Abstract
Leachables present in packaged drug products may affect the safety and quality of such products. The process by which leachables compounds are discovered, identified and quantified in a finished drug product, termed chemical assessment, consists of a series of actions which include characterizing a system’s materials of construction for their ingredients, qualifying the system by establishing its extractables and qualifying the drug product by quantifying its leachables. Part 1 of this manuscript considered the use of material characterization data (ingredients) for forecasting extractables and/or leachables profiles and illustrated the concept for a plasticized poly-(vinyl chloride) (PVC) material. Part 2 of the series includes two additional case studies (polyethylene and bromobutyl rubber) and summarizes expectations for ingredients-extractables-leachables correlations.
Introduction
Packaged pharmaceutical drug products can interact with their packaging, resulting in the movement of substances from the packaging and into the drug product. The presence of such substances in the finished drug product is of concern due to the impact that they could have on the finished drug product’s suitability for use. Thus it is necessary and mandatory that the finished drug product’s manufacturer ascertain the extent of movement and establish that the substances’ impact on the packaged drug product is within acceptable limits. So doing requires that the substances be discovered, identified and quantified in a finished drug product via a process termed chemical assessment.
Because the development of a finished drug product is a long and involved process, chemical assessment is a series of actions that together represent a logical and efficient process of risk management. This series of actions include chemical assessment; material screening and selection, the controlled extraction (simulation) study and the product assessment (migration) study.
Part 1 of this two-part series of manuscripts proposed that ingredient data could be an effective screening tool for candidate materials of construction, based on the concept that there is some relationship between ingredients in materials, extractables in packaging systems and leachables in packaged products [1]. Furthermore, it is the current state of the science that the chemical behavior of many of the more commonly utilized ingredients is well-known and well-documented. In this case, a review of a particular ingredient could produce a list of potential extractables, which consists of the combined lists of ingredients and their related substances for the system’s materials of construction. Moreover, the chemical properties of the ingredients and related extractables could be used to estimate (or forecast) the accumulation levels of the individual substances as leachables. In essence, such an assessment, based solely on knowing a test material’s composition, produces a forecasted extractables (or leachables) profile.
Correlating Ingredients, Extractables and Leachables for Materials Used in Parenteral Product Packaging: Case Studies
Currently, an Extractables and Leachables Working Group within the Product Quality Research Institute (PQRI) is extending best demonstrated practice recommendations for extractables and leachables safety assessment developed for orally inhaled and nasal drug products (OINDP) to other dosage forms, including parenterals and ophthalmics (PODP) [2]. To this end, the PODP’s Chemistry team has initiated a series of laboratory studies to produce the data and information needed to develop and support best practice recommendations. The first study, consistent with the concepts of the Chemical Assessment Triad, were controlled extraction studies performed on materials that are commonly used in packaging associated with the PODP dosage forms [2]. As the compositions of the test materials were specified prior to their characterization, the PODP study serves as an appropriate case study to illustrate the concept and utility of the ingredient – extractables correlation. Such a correlation will be illustrated for two of the materials included in the PQRI study; a low density polyethylene (LDPE) and a halobutyl rubber. A consideration of the DEHP-plasticized PVC case was reported in the first part of this series [1].
LDPE
The LDPE test material contained redundant additives that may not all be present in commercial materials but which served as sources of extractables. The composition of the LDPE test article is summarized in Figure 1. Among its formulation components are the LDPE barefoot resin itself, several antioxidants (including Irgafos 168, Irganox 1010 and butylated hydroxytoluene), a metal stearate salt as an acid scavenger, erucamide as a slip agent (among other potential functions) and Chimassorb 944 as a light stabilizer. Although the test article itself is not used in commercial applications, its composition is consistent with LDPE materials that are used in pharmaceutical applications.
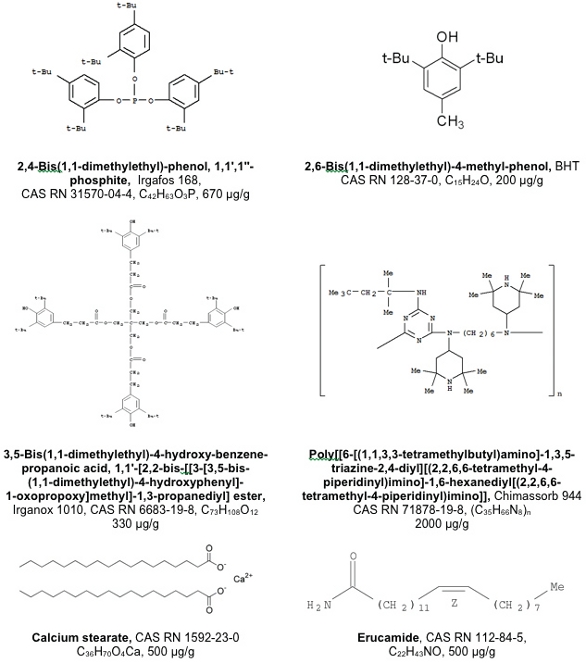 Figure 1.The Ingredients in the LDPE Test Material.
Figure 1.The Ingredients in the LDPE Test Material.Figures 2 through 5 illustrate the related substances profiles of the individual LDPE components. As LDPE materials have been characterized extensively for extractables and leachables ([3-7]), significant quantities of information about related substances are readily available and obtainable in the chemical literature. For example, the extractable substances that are typically related or attributed to the various antioxidants include their oxidative degradation products (Figures 2 – 4, [3, 8-15]). These Figures contain only the more commonly reported or more easily envisioned and justified related substances.
 Figure 2. LDPE Extractables Associated with the Irgafos 168 Antioxidant.
Figure 2. LDPE Extractables Associated with the Irgafos 168 Antioxidant. Figure 3. LDPE Extractables Associated with the BHT Antioxidant.
Figure 3. LDPE Extractables Associated with the BHT Antioxidant.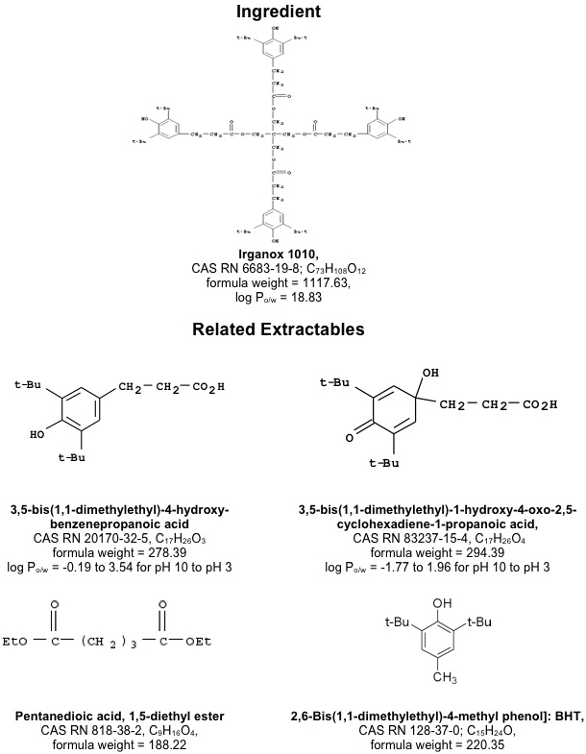 Figure 4. LDPE Extractables Associated with the Irganox 1010 Antioxidant.
Figure 4. LDPE Extractables Associated with the Irganox 1010 Antioxidant.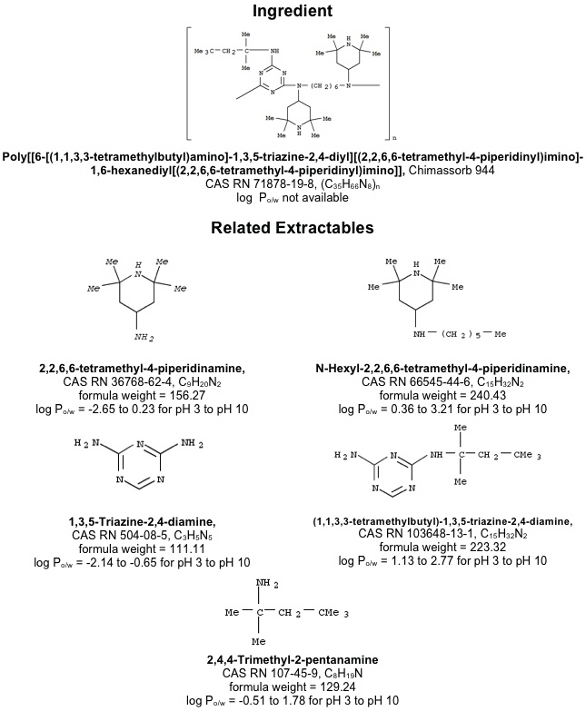 Figure 5. LDPE Extractables Associated with the Chimassorb 944 Additive. See refs. 16, 17.
Figure 5. LDPE Extractables Associated with the Chimassorb 944 Additive. See refs. 16, 17.Although generating a list of potential extractables from the compositional information is a useful endeavor, an even more useful exercise would be to forecast the semi-quantitative nature of an extractables profile that would be obtained under specified extraction conditions. To accomplish this objective, several pieces of information are relevant such as: properties of the extractable that establish their thermodynamic behavior, properties of the extractables that establish their kinetic behavior, properties of the test material that affect the migration of compounds through the material, and the total pool of the extractable in the test material. When the extraction is performed under conditions that achieve equilibrium between the test material and the extracting medium (asymptotic extraction), the kinetic factors become irrelevant.
Although quantitative extractables profiles can be mathematically generated if such quantities as total pool, partitioning behavior and extraction stoichiometry (amount of material extracted per unit volume of extraction solutions) are known, qualitative profiles can be established with less rigorous data and by less rigorous methods. Such a forecasted extractables profile is contained in Table 1. A qualitative line by line justification of the entries in this Table is as follows:
Table 1. Forecasted Organic Extractables Profile for the LDPE
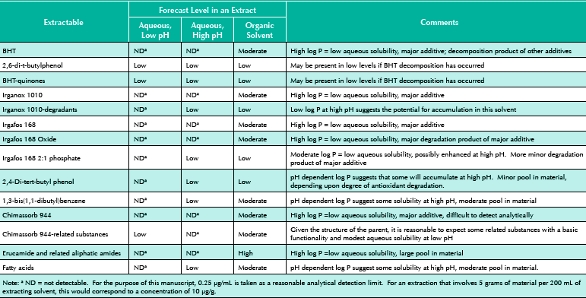
- BHT. Although present in the film at moder ate levels, this antioxidant will not be present in aqueous extracts at high levels due to its limited solubility (although BHT will accumulate at levels higher than the other, more insoluble, antioxidants). Rather, one anticipates that its more soluble oxidation products (e.g., 2,6-di-t-butylphenol and BHTquinones) will accumulate in low but measurable quantities in aqueous extracts, assuming that some BHT oxidation has occurred in the test article. BHT will accumulate at higher levels in an organic solvent due to its increased solubility in such solutions.
- Irganox 1010. This major additive will behave similarly to BHT; however, its accumulation in aqueous solutions will be so low as to be unmeasurable, given its large octanol-water partition coefficient. Due to its relati vely large pool in the LDPE, it could migrate in measurable quantities into an organic mixture. If sufficient oxidation of this antioxidant occurs, its more water-soluble Irganox degradation products could accumulate in aqueous extracts in measurable quantities. As several of the degradation products are acids, their accumulation results would depend on the pH of the extraction medium.
- Irgafos 168. This major additive will behave similarly to Irganox 1010, accumulating in aqueous solutions at unmeasurable levels based on its large octanol-water partition coefficient. This could migrate in measurable quantities into an organic mixture due to its relatively large pool in the LDPE. If sufficient oxidation of this antioxidant occurs, its more water-soluble degradation products, including its 2:1 phosphate, 2,4-di-tert-butyl phenol, and 1,3-bis(1,1dibutyl) benzene could accumulate in aqueous extracts in measurable quantities. The poorly soluble Irgafos 168 oxide will most likely not accumulate in aqueous solutions in measurable quantities.
- Chimassorb 944. Although the log Po/w for this compound was not available in the chemical literature, one anticipates that this higher molecular weight polymer would have a very limited aqueous solubility and that it would accumulate in aqueous extracts at unmeasurable levels. Alternatively, its more water-soluble degradation products could accumulate in aqueous extracts in measurable quantities. As several of the degradation products are acids,their accumulation results would depend on the pH of the extraction medium.
- Fatty acids. The fatty acids will behave in the same manner as the epoxidized fatty acids with respect the extracting solution type. The total pool of fatty acids may differ from the total pool of the epoxidized fatty acids so the levels of these two groups may be different.
- Erucamide and other amides. These are low aqueous solubility compounds whose solubilities are not pH dependent. Thus they will not accumulate in aqueous extracts at detectable levels. Because their pool in the material is moderate, they will accumulate in organic extracts to levels dictated by their individual octanolwater partition coefficients and the polarity of the organic extracting solution.
Proof of concept is accomplished when the forecast contained in Table 1 is compared with the results of actual extraction studies. As noted previously, this test material was characterized for its extractables profile through the efforts of the PQRI PODP Extractables and Leachables Working Group [2]. The resultant extracts were chromatographically characterized for organic extractables; the resulting extractables profiles are summarized in Table 2. When comparing Tables 1and 2, one notes the following:
Table 2. Organic Extractables Profile of the LDPE Material; Identified Extractables at Levels of 1 μg/g or greater.

- Most of the identified extractables in Table 2 were forecasted in Table 1, and
- In general the qualitative concentration trends forecasted in Table 1 are consistent with the semi-quantitative data provided in Table 2.
Furthermore, most of the extractables listed in Table 2 that were not directly forecast by Table 1 can logically be linked to the ingredients of the test article. Thus, it is logical to anticipate that if the individual fatty acids are both forecast and measured extractables, their methyl esters would also be present in the extracts in measurable quantities. Similarly, the presence of fatty acids and fatty acids esters other than those directly forecast in Table 1 in the extracts is logical given the composition of the test article (fatty acid salt).
Halobutyl Rubber

The rubber test article consisted of a brominated isobutylene isoprene copolymer base rubber (57.3% by weight) and contained calcined aluminum silicate, 38.2%; titanium dioxide, 1.2%; paraffinic oil, 1.2%; zinc oxide, 0.6%; polyethylene, 0.6%; Carbon black, 0.4%; calcined magnesium oxide, 0.3%; and 4,4’-dithiodimorpholine (in a polyisobutylene binder masterbatch), 0.3%. Based on this information, certain extractables can be inferred (see Table 3 [18] and Table 4). For example, it is well known that such a material will contain extractable long chain hydrocarbons and oligomeric byproducts (both brominated and unbrominated) of the material’s polymerization and/or curing process (e.g.[19]). Additionally, one can infer the structures of extractables derived from the accelerator’s accelerator (see Figure 6). Other prominent extractables associated with this test material cannot be reconciled with the material’s stated composition. These extractables can be used in the traditional process of “guessing the ingredient based on the extractables data”. Thus, for example, the presence of extractable fatty acids suggested that the rubber contained metal salts of fatty acid(s), which are commonly used as process aids in the manufacturing of halobutyl rubbers. The presence of extractable amides, oleamide and others, suggest that these materials were added to the test article at some time during its generation. The finding of antioxidants (BHT and Irganox 1010) and their know degradation products in the extracts is consistent with the common use of these compounds to stabilize halobutyl rubber materials. Reasonable
Table 3. Forecasted Organic Extractables Profile for the Halobutyl Rubber

Table 4. Organic Extractables Profile of the Rubber Material; Identified Extractablesg at levels of 1 μg/g or greater.
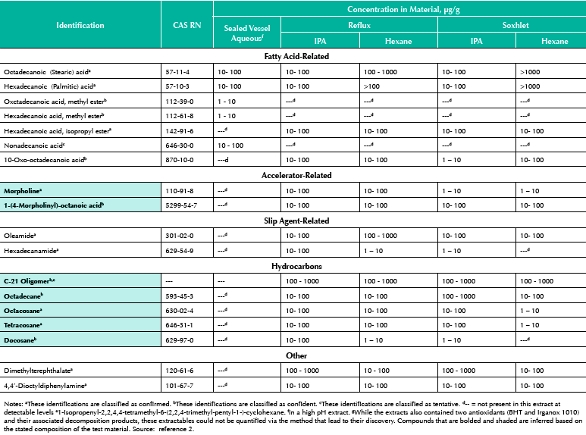
 Figure 6. Rubber Extractables Associated with the Accelerator.
Figure 6. Rubber Extractables Associated with the Accelerator.Expectations Around Ingredients, Extractables and Leachables Correlations
While one does not necessarily expect there to be a quantitative, one-to-one correspondence between a material’s ingredients, its extractables and the leachables found in a drug product that has contacted the material, it is nevertheless the case that a correlation should exist between ingredients, extractables and leachables. Incongruencies between ingredients, extractables and leachables are a clear indication that “something is not quite right”, for example:
The material contains unspecified ingredients.
- The material has been “contaminated” or “altered” during its production.
- The extractables or leachables testing is flawed.
- The “leachables” are not leachables but rather are derived from a source other than the material (for example, environmentally derived).
- There is interesting chemistry going on that should be investigated.
An extractables/leachables safety evaluation is not complete until a correlation has been established between ingredients, extractables and leachables. While the rigor with which such a correlation is established depends on the circumstances, the minimum expectation is that:
- The fate of each ingredient has been established in terms of its ability to be, or to generate, extractables and/or leachables.
- The genesis of each extractable has been established.
- The genesis of each leachable has been established.
Only when such a correlation has been established can the leachable be controlled in the final packaged drug product by controlling the composition of the incoming raw materials.
References
- Jenke, D; Ruberto, M. Using the correlation between material composition and extractables and leachables to forecast extractables and/or leachables profiles. Pharm Outsourcing. Vol 15, Issue 1 (2014).
- Jenke, D. et al. Extractables characterization for five materials of construction representative of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Tech. 76(5): 448-511 (2013).
- Ruberto, M.A.; Paskiet, D.; Miller, K. Chemical and physical attributes of plastics and elastomers: Impact on the extractables profile of container closure systems. In Leachables and Extractables Handbook: Safety Evaluation, Qualification, and Best Demonstrated Practices Applied to Inhalation Drug Products (Wiley,Hoboken, NJ, USA, 2012, pp. 185-216).
- Jagnandan, I.; Dunnn, H.; Ambrosio, J.; Gilbert, S.G. Isolation and identification of 3,3’,5,5’-tetrabis (tertbutyl) stilbenequinone from polyethylene closures containing titanium dioxide and butylated hydroxytoluene. J. Pharm. Sci. 68: 916-917 (1979).
- Azuma, K.; Tanaka, Y.; Tsundo, H.; Hirata, T. Ishitani, T. Effects of film variety on the amounts of carboxylic acids from electron beam irradiated polyethylene film. Agric Biol Chem. 48: 2003-2008 (1984).
- Helmroth, I. E.; Bekhuis, H. A. M.; Linssen, J. P. H.; Dekker, M. Direct measurement of additive migration from low-density polyethylene as a function of space and time. J. Appl. Poly. Sci. 86, 3185-3190 (2002).
- Akapo, S. O.; McCrea, C. M. SPME-GC determination of potential volatile organic leachables in aqueous-based pharmaceutical formulations packaged in overwrapped LDPE vials. J Pharm Biomed Anal. 47(3): 526-534 (2008).
- Jenke, D. Extractable/Leachable substances from plastic materials used as pharmaceutical product containers/devices. PDA J Pharm Sci Technol. 56(6):332-371 (2002).
- Hammond, M. et al. Identification of a leachable compound detrimental to cell growth in single-use bioprocess containers. PDA J Pharm Sci Technol. 67(2):123-134 (2013).
- Buchberger, W.; Stiftinger, M. Analysis of polymer additives and impurities by liquid chromatography/mass spectrometry and capillary electrophoresis/mass spectrometry. Adv Polym Sci. 248: 39-68 (2012).
- Burman, L.; Albertsson, A.C.; Holund, A. Solid-phase microextraction for qualitative and quantitative determination of migrated degradation products of antioxidants in an organic aqueous solution. J Chromatogr A. 1080: 107-116 (2005).
- Zweifel, H. Plastics Additives Handbook, 5th ed. Hanser, Munich, 2001.
- Klemchuk, P.P.; Horng, P-L., Transformation products of hindered phenolic antioxidants and color development in polyolefins. Polym. Degrad. Stab. 34(1-3): 333-346 (1991).
- Schwetlick, K. Mechanisms of antioxidant action of organic phosphorus compounds Pure & Appl. Chem. 55(10): 1629-1636 (1983).
- Klemchuk, P.P.; Gande, M.E. Stabilization mechanism of hindered amines. Polym. Degrad. Stab. 22(3): 241-274 (1988).
- Haider, N.; Karlsson, S. Loss of Chimassorb 944 form LDPE and identification of degradation products after exposure to water, air and compost. Poly Degr Stab. 74(1): 103-112 (2001).
- Coulier, L.; Orbons, H.G.M. Analytical protocol to study the food safety of (multiple-) recycled high-density polyethylene (HDPE) and polypropylene crates: Influence of recycling on the migration and formation of degradation products. Poly Degr Stab. 92(11): 2016- 2025 (2007).
- Janssen, R., Extractables / Leachables from Pharmaceutical Rubber Components for Parenteral Applications, PharmaEd Extractables and Leachables Conference, May 3-4, 2010, San Francisco.
- Wong, W.K. Impact of elastomer extractables in pharmaceutical stoppers and seals; material supplier perspective. Rubber World. 240(3): 20-29 (2009).
Dr. Dennis Jenke is a Baxter Distinguished Scientist at Baxter Healthcare Corporation. He has published extensively in the areas of analytical chemistry, environmental science and material/solution compatibility. He serves as an expert reviewer for pharmaceutical and analytical journals, is a member of professional and standard-setting organizations whose charter is to establish best demonstrated practices for material/solution compatibility and is a frequent speaker on the subject of material/solution compatibility. Dr. Jenke authored the book “Compatibility of Pharmaceutical Solutions and Contact Materials; Safety Considerations Associated with Extractables and Leachables”.
Dr. Michael Ruberto is the President of Material Needs Consulting, LLC which provides consulting services to manage the development and commercialization of medical devices and packaging, emphasizing material selection, extractables and leachables, and supply chain management. He is an active member of organizations that have developed best practices for characterizing and evaluating container closure systems and packaging including the PQRI Orally Inhaled and Nasal Drug Product E&L Working Group, the PQRI Parenteral and Ophthalmic Drug Product E&L Working Group and the United States Pharmacopeia (USP) Packaging and Storage Expert Committee.