Introduction
Twenty years ago, the risk to drug substance/product quality due to temperature excursions during shipping was not generally appreciated. A series of studies led to several papers in USP PF which documented some of the risks1-4. In response, increasing regulatory requirements related to supply chain temperature controls have led to inconsistent approaches to what types of data are required and, once data is developed, which sections within the filing (eCTD) to submit these types of data.
This paper will list types of data that is now being requested to show supply chain temperature controls and recommend areas within the filing to submit these similar data. However, the location within the filing is a recommendation, realizing that data generated for a specific reason may be suited to another section better than the example recommendations. It is paramount that the data filer knows what the data will be used for – to better understand why one section is better than another. It is also recommended that same data sets not be filed in multiple locations – but that they be cross-referenced.
Data Type Discussion
The purpose of formal stability studies is to develop supporting data for shelf-life determination – at a specific storage temperature range (e.g. 15-25°C for 24 months). Stability studies/conditions are developed based of the International Conference on Harmonization (ICH) section Q1A, which also requires accelerated stability testing5. Formal and accelerated stability studies can vary dependent on which international stability zone that the product will be marketed in6. Above and beyond standard ICH Q1A, firms are submitting the following stability data including extreme excursion temperatures and temperature cycling data as depicted in Table 1 below7,8.
Table 1. Example Stability table (include Formal, Stress (both are ICH) and Temp Excursion/Cycling) for solid oral dosage forms intended to be stored at room temperature.

Data Type Discussion and eCTD Section
Regulators are increasingly asking for additional data to support supply chain controls as described in the paragraphs below.
- Temperature Excursion and Temperature Cycling Stabilitydata: This type of data is above and below labeled storage conditions (formal ICH Q1 stability). The data generated from these studies can be used to develop a “stability budget” and are used to support excursions that may be seen during storage and transportation that are outside of storage label claim.
a. It is recommended that this type of data be filed in the Stability sections (S.7 and P.8) – see Table 2 for an example of filing temperature cycles stability data - Shipping Validation Studies: The stability data set developed for a product along with the transport process characteristics (mode, route, transit time, ambient temperature conditions, etc.,) should be used to define the appropriate level of temperature controls (e.g. thermal containers) and monitoring to ensure that product quality is maintained during the transport process. The transport process selected should be based on a documented risk-analysis and may be defined as requiring specific temperature controls and/or monitoring, continuous verification or qualification/validation.
a. It is recommended that this type of data be summarized and filed in the Process Validation sections (S.2.5 and P.3.5) - Container closure integrity (CCI): The container closure integrity, or CCI, plays a critical role in the preservation of a drug product by ensuring that a packaging system provides the necessary protection required to meet physicochemical and microbiological label-claim specifications through expiry. CCI testing performed during stability testing should include the generation of data to support distribution activities. During distribution, a packaging system can be challenged by temperature, humidity, pressure and shock/ vibration, which can impact on the packaging system ability to protect the product (e.g. microbial contamination, product loss, loss of gas headspace).
Examples
- Elastomeric closures have been found to lose their viscoelastic properties during exposure to ultra-cold temperatures (≤ -80°C) to such an extent that gas influx into stoppered vial packages may occur.
a. Recommendation: Thermal Stress Studies - Blister packaging, made of various polymeric materials, can lead to increased moisture permeation into the packaging system when exposed to increasing temperature and humidity.
b. Recommendation: Thermal Cycle Studies - The transportation of prefilled syringes by air has shown that reduced pressure could induce plunger movement and provide a pathway for the ingress. Temperature changes can also have an impact on the quality of the product.
c. Recommendation: Thermal Cycle Studies along with Pressure Studies
Table 2. Recommended/Potential eCTD sections for data types
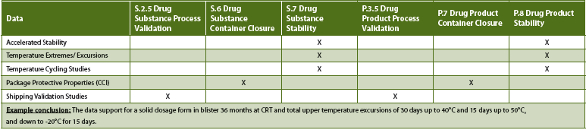
CCI testing is packaging system specific and not product specific. Initial CCI testing can be performed using a surrogate and need not be performed for each product but the expectation is that it be performed on stability where warranted.
It is recommended that this information be filed in the Packaging sections (S.6 and P.7) and cross reference the stability budget for distribution related issues. Sections S.6 and P.7 contain the description of the closure system but do not include the CCI validation information. Since CCI is relevant to the thermal stress/cycling studies, to the distribution studies and to packaging definitions, adding validation and qualification information to S.6 and P.7 may not be the most appropriate location, however if a specific section is chosen as a best fit, cross referencing to the validation and product stability is a good idea.
Summary
Recommendations on where to file specific data in support of supply chain temperature controls are listed in Table 2.
Conclusion
There are additional data types being requested by global regulators in the eCTD to support GDP (Good Distribution Practices) – related to transportation temperatures. This paper describes the stability studies that go beyond ICH, how some have been modified/expanded and new stability studies (e.g. temperature cycling) – and why they are being requested and then recommend where those types of data set maybe filed. These are recommendations on where to file specific data sets, and regardless of where data is filed, cross referencing to appropriate sections is a good practice. The guidance proposed here is to assist with global temperature management.
References
- Okeke, C.C., Bailey, L.C., Medwick, T., and Grady, L.T., “Temperature Fluctuations During Mail Order Shipment of Pharmaceutical Articles Using Mean Kinetic Temperature Approach,” Pharmacopeial Forum, 23(3) May-June 1997, page 4155-4182.
- Okeke, C.C., Bailey, L.C., Lindauer, R.F., Medwick, T., and Grady, L.T., “Evaluation of the Physical and Chemical Stability of Some Drugs WhenExposed to Temperature Fluctuations During Shipment,” Pharmacopeial Forum, 24(5) Sept.-Oct.1998, page 7064-7073.
- Okeke, C.C., Bailey, L.C., Medwick, T., and Grady, L.T., “Temperature and Humidity Conditions During Shipment in International Commerce,” Pharmacopeial Forum, 25(2) Mar-April1999, page 7949-7959.
- Okeke, C.C., Watkins, J.W. III, Williams, W., Medwick, T., Bailey, L.C., and Grady, L.T., “A Study of the Temperature and Humidity Variations in the Shipping and Distibution of Anthrax Vaccines,” Pharmacopeial Forum, 26(3) May-June 2000, page 865-882.
- ICH Q1A(R2), Stability Testing of New Drug Subtances and Products, 2003.
- WHO Expert Committee on Specifications for Pharmaceutical Preparations - WHO Technical Report Series, No. 863 - Thirty-fourth Report, Annex 5 Guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms, 1996.
- PDA TECHNICAL REPORT NO. 53, STABILITY TESTING TO SUPPORT DISTRIBUTION OF NEW DRUG PRODUCTS, 2001.
- TECHNICAL REPORT NO. 39. COLD CHAIN GUIDANCE FOR MEDICINAL PRODUCTS: MAINTAINING THE QUALITY OF TEMPERATURE-SENSITIVE MEDICINAL PRODUCTS THROUGH THE TRANSPORTATION ENVIRONMENT, THE PDA JOURNAL OF PHARMACEUTICAL SCIENCE & TECHNOLOGY, SEPT-OCT 2005.
Dave Ulrich is the QA Director – Supply Chain compliance for Abbvie’s Global Supply Chain. His responsibilities include standard-ization & optimization of supply chain quality systems (cGDPs), compliance for supply chain temperature management, ePedigree Track-n-Trace, serialization, and import export activities. He has been at Abbott (now Abbvie) 28 years in various roles in Labs, plant maintenance, manufacturing QA, and engineering. Previous to Abbott he was lab manager at Cabrini Hospital in Chicago.
Rafik H. Bishara, Ph.D., Is the current Chair of the Pharmaceutical Cold Chain Interest Group (PCCIG) within the Parenteral Drug Association (PDA). Dr. Bishara has become one of the most respected figures in the pharmaceutical supply chain distribution sector, following a distinguished 35 year career with Eli Lilly & Co. as Director, Quality Knowledge Management and Technical Support. Dr. Bishara has authored numerous articles, and technically advised several organizations on Good Cold Chain and Temperature – Controlled Management. His current focus include Supply Chain Integrity and Security. Contact: [email protected].
Colleen Hutter has been in Packaging at Merck for more than 18 years. She has designed and commercialized primary, secondary and tertiary packaging systems for drug substance, drug product and finished goods for solid oral dosage, vaccine and biologic products throughout her career. Colleen currently leads a team of subject matter expert engineers who design, develop and qualify temperature-controlled shipping systems for the Merck network. Colleen is a Six Sigma Black Belt and earned a BS in Packaging from Michigan State University. She currently resides in Eastern Pennsylvania with her husband and two young children and is active in her community.
As a Regulatory Affairs Director at Abbvie, Rich Poska focuses on strategic CMC initiatives and mature product strategy. He earned his B.S. degree in Pharmacy from the University of Illinois College of Pharmacy and has worked with solid dosage forms at both Searle Laboratories and The Upjohn Company before joining Abbott. His 20 years of technical experience includes solid dosage form research, production troubleshooting, process improvement and support, harmonization of excipients and processing, application of statistics for manufacturing controls, Puerto Rico liaison and FDA inspection administrator. He is internationally known for his presentations and publications on the application of microwave drying to pharmaceutical processing. He chaired the SUPAC Equipment Equivalents Steering Committee for the International Society of Pharmaceutical Engineers (ISPE) which won the Vice-President’s National Performance Review “Hammer Award” for building a government that works better and costs less. Rich has also chaired PhRMA’s Technical Leadership Committee and Drug Product Technical Committee and has represented ISPE and PhRMA on the Drug Product Technical Committee of the Product Quality Research Institute.
Dr. Desmond G. Hunt is a Sr. Scientific Liaison in the Department of Standards Development (DSD) at the United States Pharmacopoeia. For the 2010-2015 revision cycle, he is responsible for assisting USP Expert Committees, Packaging, Storage and Distribution and Dosage Forms, in the development and revision of USP Standards. Dr. Hunt has over 15 years of research experience and prior to joining USP, in 2005, was a Research Fellow at the National Institutes of Health, Bethesda, MD, USA. Dr. Hunt’s expertise is in the areas of pharmaceutical packaging, good distribution practices, parenteral drug products, ophthalmic preparations and particulate matter analysis. He has published extensively nationally and international and has taught courses on various pharmaceutical topics around the world. Currently, he is a member the Product Quality Research Institute Container-Closure and Extractable and Leachable Working Groups; APEC– Global Drug Integrity and Supply Chain Security – Roadmap Committee and the Center for Disease Control Committee on Vaccine Storage. He obtained his Master of Science and Doctoral Degree from the University of Texas at Austin, USA.
Riekert Bruinink is a Senior GMP/GDP inspector at the Dutch Healthcare Inspectorate. He was Chairman of the PIC/S GDP Working Group and a member of the EMA GDP Drafting Group This group was responsible for making the new EU GDP Guidelines. He is member of the USP Packaging & Distribution Expert Committee .
Michael English is an Associate Director of Engineering, Packaging Technical Operations for Merck & Co., Inc. He has been in Technology for 15 years with responsibilities including the development and qualification of thermal protection systems, cold chain management, and supply chain excellence. Michael has been with Merck for 26 years with roles in Quality, Logistics, and Technology.
Arminda O. Montero is Global Supply Chain QA Manager, Supply Chain and Commercial Quality. She has over 15 years of experience in the pharmaceutical industry. In her current role, she leads a global team with responsibility for supply chain integrity, distribution and logistics, and temperature control management strategic quality initiatives. Prior to this role, she held positions in engineering, manufacturing operations management and operational excellence. Arminda holds a Bachelor of Science degree in Chemical Engineering from the University of Illinois at Urbana-Champaign