by Joe Cobb, R. Justin Brett, Michael D. Ruff, Anthony Berry, Robert Epps
OBJECTIVE
To improve robustness of a core tablet for a novel modified release oral solid dosage form using Quality by Design (QbD) principles.
General Background
As stated in the International Conference on Harmonisation Harmonised Tripartite Guidance on Pharmaceutical Development, ICH Q8 (R2), “The aim of pharmaceutical development is to design a quality product and its manufacturing process to consistently deliver the intended performance of the product.”1 Several tools are available as guidance issued by FDA such as “Quality Systems Approach to cGMP Manufacturing”2 that includes ideas such as Quality by Design (QbD) in the development process. This guidance, amongst others, lay the framework for expectations of regulatory reviewers in their examination of client submittal documentation.
This project involved first the technical transfer of a formulation and process of a novel modified release oral solid dosage form (i.e. an active core tablet with an a series of coatings that modified the release and delivered additional quantities of the same active ingredient) that was originally manufactured at a relatively small scale. The next step was a scale-up of the process to commercial scale but due to time constraints not all of the unit operations had been completely optimized. Upon discovering processing issues such as relatively high friability and low breaking strength of the core tablets the client agreed to proceed with optimization utilizing principles of QbD.
The first step of the QbD process was to establish a Quality Target Product Profile (QTPP) for the core tablets. The second step was to determine the Critical Quality Attributes (CQA) of the core tablets. The third step incorporated a risk assessment exercise to identify the Critical Processing Parameters (CPP). The final step involved drafting an informal Design of Experiments (DOE) to determine optimal settings of the CPP’s for the critical high shear wet granulation process.
METHODOLOGY
Materials
Active Pharmaceutical Ingredient (API) X, (Supplied by client)
Microcrystalline Cellulose, NF/EP (Avicel ® PH 102 and PH 200, supplied by FMC)
Partially Pregelatinized Starch, NF (Starch 1500® supplied by Colorcon)
Croscarmellose Sodium, NF/EP (Ac-Di-Sol ® SD-711 supplied by FMC)
Magnesium Stearate, NF/EP, (Non-bovine, supplied by Mallinckrodt)
Various reagent grade chemicals and solvents to execute required analytical testing
Manufacturing Equipment
TK Fielder PMA-100 High Shear Granulator w/ Pressurized Spray Pot
O’Hara FBDG-30 Dryer with 100 liter bowl
Fitzpatrick Fitzmill, Model M
15 ft3 Tote Blender
Manesty Unipress equipped with 0.25” round standard concave tooling
Analytical Equipment (Physical testing only)
USP <1216> Compliant Tablet Friability Tester
USP <1217> Compliant Tablet Breaking Force Tester
Background Formulation Information
The core tablet formulation listing ingredients, functionality and quantitative amounts is given in Table 1 at the top of the next column.

Based on the relatively late stage in the development cycle, the client agreed that neither the core tablet formulation nor the unit operations used in its manufacture could be changed. See Figure 1 below for a flowchart of the core tablet manufacturing process.

In-process data gathered during the compression process indicated that the core tablets had excellent weight control. However, even though the core tablets had satisfactory friability to pass USP <1216> (Not more than 1.0% 3) the team agreed that they would not be able to withstand the rigors of downstream coating in a standard side-vented coating pan. The team further agreed that optimization on the entirety of the core tablet manufacturing process was necessary using QbD principles.
The first step of the optimization process using QbD principles was to establish a Quality Target Product Profile (QTPP) for the core tablets themselves. Based on the phase of work involved several elements of the QTPP had already been established, such as the fact that the final dosage form was going to be a modified release product with a novel coating scheme to allow for immediate and delayed release of X. However a more formalized declaration of QTPP specific to the core tablet itself had not. See Table 2 below for the QTPP for the core tablets themselves.
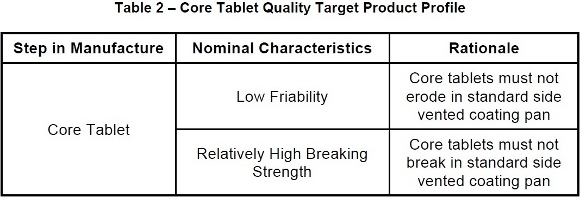
The next step of the optimization process using QbD principles was to utilize the QTPP above to derive a set of Critical Quality Attributes (CQA) of the Core tablets. The CQA would be based on empirical evidence derived from previous experimentation as well as similar experiences with other products. See Table 3 below for the CQA of the core tablets.

Using the attributes given above the team organized a set of Critical Process- Parameters (CPP) utilizing a risk-based approach to all of the upstream unit operations. This was based on previous experience with this project as well as other similar dosage forms with equivalent or similar equipment trains. See Table 4 for CPP using a riskbased model of potential effects on CQA from above.

To build on previous experiments already performed, an abbreviated Design of Experiments consisted of examination of only two of the CPP’s that had not been already optimized. The two variables to be examined were granulating liquid amount and post-spray wet-massing time. An full factorial experimental design based on two factors with three settings (32) was agreed to, but with one experiment left out (Medium Liquid Amount, Low Wet Mass Time) because that had been evaluated in previous work and found to give unsatisfactory tablet physical characteristics. Factor levels were defined as such:
Liquid Amount: Low = 8.500 kg, Medium = 9.000 kg, High = 9.500 kg
Wet Mass Time: Low = 2 minutes, Medium = 4 minutes, High = 6 minutes
See Table 5 below for factors for the abbreviated experimental design.

RESULTS
CQA’s listed in Table 3 were evaluated for each experiment in the DOE and compared to previously generated data for acceptability and improvement. See Table 6 below for a summary of results from each experiment.

Discussion
Based on the results, high liquid amount and low wet mass time (Experiment #6), yielded the best blend and tablet characteristic responses. All experiments showed acceptable flowability and uniformity of dosage units to satisfy the requirements as stated in CQA. Based on these findings the conditions given in experiment # 6 are considered optimal.
CONCLUSION
Robustness of the core tablet was substantially improved by following QbD principles and performing an informal DOE based on these findings.
1 – ICH Q8 (R2), Revision from August 2009. Page 1
2 – FDA Guidance Quality Systems Approach to CGMP Regulations, September 2006
3 – Current USP (35), General Chapter <1216> “Tablet Friability”
4 – Current USP (35), General Chapter <905> “Uniformity of Dosage Units