In this era of mergers and acquisitions, collaborative manufacturing partnerships, and increasing numbers of outsourcing deals, the biopharmaceutical industry faces the challenge of adopting the best externalization strategies that are financially sound, and that ensure adequate oversight without hindering a Contract Manufacturing Organization’s (CMO’s) operational efficiency. In pre-commercial outsourcing, quicker to clinic is critical, without sacrificing quality, and preferably, at lower costs!
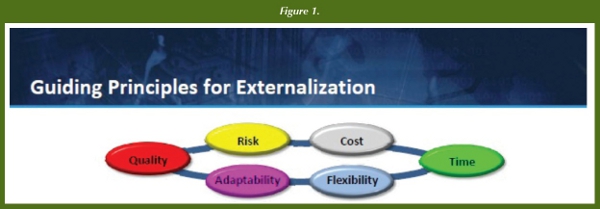
The Biotherapeutics & Vaccines Outsourcing Unit within Pfizer has developed Guiding Principles for Externalization to ensure that is selected the best CMO for technology transfer, and any potential risks with outsourcing the production and control of clinical supplies are minimized. There are six key principles: Quality, Risk, Cost, Time, Flexibility, and Adaptability.
Quality is typically influenced by the complexity and novelty of the process for manufacture - from a single recombinant protein expressed in E. coli for instance, to a multiple-antigen (try 23-mer!) polysaccharidetoxoid conjugate in a vaccine drug product. Risk can be associated with several areas of concern – from regulatory strategy, to patent state, to trade secret, and core know-how. Cost is straight forward, but can be complicated by licensing and royalty burden concerns, number of fulltime equivalents necessary for complex projects and, (although not typical for clinical supplies), cost of goods or COGs. Time is time – minimize the time to Proof-of-Concept (POC) and maximize the number of Proof-of-Concept trials in a year. Flexibility points to scheduling, with resources and technology platforms facilitating movement of projects; “platformed” processes minimize duration of technology transfers. Finally, Adaptability, aka “Cultural Fit”, ensures maintenance of good vendor relations to meet shifting environment and project needs, typical of the clinical work space.
As part of the sourcing decision process for a Pre-POC project, there are several “Make” vs. “Buy” options that are taken into consideration. Regardless of the modality, i.e., Drug Substance (DS), Drug Product (DP), or Analytics, questions of whether the project reflects core abilities or not, are essential to siting decisions. Cell bank development and manufacture, media development, purification development and optimization, conjugation development, formulation development, and critical or complex assay development and testing, are just a few examples of core capabilities that would influence a “Make” decision, i.e., keep the work in-house. For unique facility concerns like viral vaccines and vectors, antibody-drug conjugates, or routine production processes, and compendial / stability testing, “Buy” decisions are favored, i.e., outsource.
In order to support pre-commercial externalization needs, we have developed vendor networks that are technology-driven, and are complementary to the capabilities of our own facilities. We have identified CMOs that are experts in single modalities (Functional Service Model) or those that have “soup-to-nuts” capabilities (Integrated Service Model). As with any model, there are always pros and cons. For the Functional Service Model, we are able to leverage the CMO’s strength and expertise in a specific modality space to our advantage, with a good chance of getting to the clinic as speedily as possible. The downside to this strategy is that the supply chain is more complex, and the costs could be higher, since the production and testing activities could be split among two or three different CMOs for DS, DP, and analytics. Having one CMO perform all three functions would be ideal – thus, the Integrated Service Model. There would be only one technology transfer occurrence and one oversight team. Developing a strong relationship or bond between the transferring and receiving organizations is crucial to a successful contract. A negative aspect to this is the reliance on a single provider, and if you have not done your homework and exercised due diligence in screening your vendors, it can end in disaster!
For this reason, the vendor screening process becomes a critical component in outsourcing. Typically, we start by issuing a Request for Information (RFI) to a pool of vendors which have the requisite capabilities in a specific modality. This is a high-level vendor screen, looking at process and analytical development capabilities, DS production and testing capabilities, DP production and testing capabilities, and stability studies for DS and DP, to name a few. From the RFI analysis and deliberation, we develop a “short list” of CMOs to whom we send a formal Request for Proposal (RFP). We expect a more detailed response, replete with tight cost estimates, which become the basis for generating a Master Service agreement. CMOs are scored and ranked based on pre-determined weighted criteria.
Financial stability is not weighted, but can be a “Go / No Go” factor. Here, you want to ensure that the CMO will remain solvent for the life of your contract. Technology transfer is a very costly process and of course, time-consuming, so you do not want to be caught in the middle of a project with a CMO that fi les a “Chapter 5”! For Company Profile (20%), a CMO that already has experience with us or with any of our business units becomes an advantage for obvious reasons. Geographic location of the CMO plays an important role as well – proximity to the transferring organization provides lower costs for travel and oversight. Turnover rate of a CMO is reflective of its culture, which can lend an important hand in the well-being of their personnel. Do they like their jobs? Are they happy? Management (20%) points to the overall management of the contract or project, as well as the relationships between different units at a CMO, i.e., upstream cell culture and downstream purification groups. Internal squabbles during hand-offs are detrimental to a project’s progression. An experienced CMO typically has an adequately resourced Project Management group that facilitates communications between the transferring and receiving organizations. This is NOT a function for the Subject Matter Experts (SMEs), who focus on the technical aspects of the project. A well-managed contract is very important to its success! Quality (30%): The ratio of the science staff to the Quality Assurance staff demonstrates the level of attention a CMO pays to this attribute. are quality systems in place? Is there a clear investigation and CaPa procedure? Is the technical staff properly trained and is this documented? These are just a few questions whose responses reflect how well embedded Quality is in a CMO. Also of importance is the audit history of a CMO – audits by Boards of Health like the FDa or the EMEa, or even audits by potential clients. Few observations from numerous audits is a good thing! Capabilities & Experience (30%): Depending on the complexity of a project that will be outsourced, this may become the driver for the selection of a CMO. Clearly, proficiencies in a particular modality will facilitate technology transfer, and can even lend to process improvements and increased yields - a “win-win” situation for both client and CMO. And of course, the state-of-the-art process and analytical equipment in a well-designed facility is “icing on the cake”! Finally, although also not weighted, Cost Competitiveness can become the tiebreaker, when faced with choosing between CMOs that are equal in all of the aforementioned selection criteria.
After narrowing down the choices to two or three potential CMOs post-RFP, the next steps are also just as important. A site visit from our SMEs, typically facilitated by an outsourcing colleague, follows. This face-to-face meeting between the two organizations can either leave you with good lasting impressions or a “bad taste in the mouth”. This due diligence activity also ensures us that those gorgeous, glossy pictures of facilities and equipment either in brochures or in websites do exist, and are as described! a Technical Evaluation Report is prepared, with a recommendation to go forward with a comprehensive Quality audit – or not. After successful completion of a 2- to 3-day Quality assessment by our professional auditors, with or without a Quality Person present, the CMO is now deemed to be “acceptable” for use. Then the business negotiations can begin, and Quality and Technical agreements can be generated, paving the way to the initiation of technology transfer. This, in itself, is another multi-faceted process, with bells and whistles in place, for discussion in another article.
There are many definitions of SUCCESS, but for us in the pre-commercial outsourcing landscape, it is simple - “Quicker to clinic is key, at reduced costs, without sacrificing quality!” To achieve success in every contract negotiated and managed, it is necessary to work hard upfront in de-risking it. Indeed, outsourcing remains a risky business, but there are many strategies to minimize risk.
Dr. Firelli Alonso-Caplen is a Senior Director at Pfizer, Inc. She heads the Biotherapeutics and Vaccines Outsourcing group within the BioTherapeutics Pharmaceutical Sciences organization of PfizerWorldwide Research and Development. Fi has more than 28 years of combined experience in research, development, and cGMP production of biological products and vaccines, and more than 8 years experience in outsourcing, project / contract management, and technology transfer to qualified third parties. Her areas of expertise include viral vectors and vaccine development, biotherapeutics and vaccine process development and cGMP production, project management, technology transfer, outsourcing, and budget and operations.
She obtained her Ph.D. in Microbiology from the University of Alabama in Birmingham, followed by postdoctoral research at the U.S. Army Medical Research Institute for Infectious Diseases, Sloan-Kettering Institute for Cancer Research, and Rutgers University Center for Advanced Biotechnology and Medicine. Prior to working for Wyeth / Pfizer in 1996, Fi was at The Salk Institute (Government Services Division), a vaccine contract manufacturer for the U.S. Army.