Introduction
Since the early 1980s, biotechnology products have shaped the pharmaceutical industry. A large number of monoclonal antibodies and therapeutic proteins have been approved and are predicted to be the major source of revenue in upcoming years for the industry [1-5]. Due to their ability to properly fold and glycosylate these large molecules, mammalian cells are the expression system of choice for recombinant therapeutic proteins [7-10]. Manufacturing scales up to 25 m3 operated in a batch, fed batch, or repeated batch mode followed by sequence of chromatography, filtration and concentration steps represent the state of the art technology [11,12]. A typical antibody production process is shown in Shukla and Thömmes 2010 [12].
However, the industry is facing numerous challenges in upcoming years. A review of data from the 1950s to 2008 indicates a trend toward stagnant R&D outputs and significantly increased development cost, representing a major threat for most innovation-driven pharmaceutical companies [5]. In response to this threat, several measures to improve the competitive edge along the value and supply chain have been successfully executed, such as lean and six sigma principles. Biopharmaceutical manufacturing in the 21st century is required to be agile, nimble, and flexible in order to adapt quickly to a changing environment and guarantee uninterrupted supply of high quality therapeutics with acceptable financial performance. Optimal manufacturing costs, asset utilization, and supply chain risk mitigation are key success factors in delivering value to the industry in years to come. Manufacturing process technology transfers, when executed effectively, can be a strategic enabler to the modern pharmaceutical industry in meeting its future objectives. In the past, technology transfers were largely driven by the product lifecycle, from clinical supply to commercial launch. Today, additional strategic aspects driving technology transfer have come into play, such as supply chain optimization, market access, capacity utilization, sourcing/ contract manufacturing considerations, risk mitigation, and divestment. In addition, increasing robustness, “upgrading” control strategies, adapting older processes to current state-of- the-art manufacturing platforms, and mitigating risks, without compromising product quality and process consistency is essential in the industry. Post-approval lifecycle management is therefore an essential part, from both a regulatory and business perspective. The implementation of new process versions driven out of process development or the implementation of mature technologies in existing processes (post-approval change) to improve commercial manufacturing processes in alignment with regulatory expectation is an opportunity during tech transfers.
Therefore, technology transfer has become an increasingly vital business enabler, as efficient execution is a critical success factor for global entities.
Although all technology transfers are generally complex, the transfer of mammalian cell culture processes is particularly challenging when compared to the transfer of a chemical manufacturing process. In general, this technical complexity is due to the unique characteristics of mammalian cells, the sensitivity of therapeutic protein products and the complexity in analytics defining product quality and process comparability between sites. Although each transfer is different, some common rules for technology transfers can be formulated in order to drive efficiencies and mitigate risks. This article will describe several success factors from an organizational and management perspective, equipment and process knowledge, risk management standpoint, and the integration of these. Case studies will illustrate tools and examples of the successful mitigation of risks, knowledge increase, and process improvements.
Business Process Management and Governance
The execution of a transfer involves several steps and should follow a pre-determined business process, including the description of major activities, milestones, and deliverables. Figure 1 shows a high-level, exemplary tech transfer business process which will be explained in more detail.
A sourcing decision typically precedes the start of a tech transfer. The first step is the gathering of data to prepare a sound sourcing decision, including business cases and risk assessments. The data analysis triggers a “make vs. buy” decision and a transfer project is initiated.

Figure 1. Exemplary tech transfer business process13
The first steps in the planning phase of the transfer are the appointment of the leader and a transfer team, training in roles and responsibilities, and creation of a draft transfer master plan. Various subject matter experts and stakeholders need to be involved in the transfer due to the crossfunctional nature and complexity of the project. An integrated approach for avoiding disconnects is important to bypass delays or conflicts, and to ensure efficient knowledge management. Clear roles and responsibilities,decision-making rights, and escalation mechanisms need to be defined and reflected in the organizational setup. Typically, a steering committee, a management committee, and technical subject matter expert teams comprise the governance structure to create transparency and efficiency in decision making. Figure 2 shows an exemplary setup for a tech transfer governance structure. The steering committee might report into executive level governance bodies, for example, product lifecycle teams or technical development committees. Intercompany teams for decision making and escalation are desirable in cases of transfer to a contract manufacturing organization, leading to joint ownership and supplier integration.

Figure 2. Exemplary governance structure
The steering committee typically gives strategic direction, approves deliverables, reviews progress, allocates resources, approves project changes, and serves as an escalation body for project conflicts.
The members of the team have to be from an appropriate level of the organization and hold adequate decision making rights. The next level can be defined as a management team. This team owns the execution and realization of the transfer. Their focus is partly strategic, yet primarily tactical. Detailed project planning, technical subject expert guidance, project coordination, and end-to-end realization of the process comprise the key responsibilities of the management team. The cross-functional team consists of supply chain and logistics functions, regulatory affairs, engineering, quality assurance and the technical project leads from the donor and receiving sites, as well as a quality control leader for assay transfers. In order to provide sufficient information and granularity on a local level, some of these functions have local sub teams responsible to deliver according to the timelines. A robust governance structure is one major success factor during the transfer. Unclear roles and responsibilities, opacity in decision making, or lack of information flow can lead to timeand resource-intensive delays or failures of the tech transfer. Raw material supply chain, including release testing, assay transfers, document and data transfer (knowledge management), facility modifications, process adaptations, project management, and risk management are some of the most important aspects that need to be addressed for success.
In the next phase of technology transfers, process data compilation begins to describe and assess the manufacturing process. A predefined list of required documents should exist as a template. Here, detailed process description, analytical assays and data, production equipment details, historical process data and will be transferred. Therefore, excellence in knowledge management in alignment with regulatory expectations is of crucial importance during the lifecycle, from process and product development, to ongoing commercial production after approval (Figure 3). This will be the basis for gap analyses, facility fit assessments, and site-specific process descriptions, including the identification of bottlenecks, capital expenditure needs and process adaptations needed.

Figure 3. Exemplary concept for knowledge management aspects13
The verification of understanding and completeness of the documents is of crucial importance. Communication and relationship build-up between the key players of the interacting teams are important to guarantee seamless execution. In order to finalize the master transfer plan, detailed gaps from a process, facility, analytical, and regulatory perspective need to be identified and assessed via a stringent risk-management process and embedded into the change control process. Strong interaction between all involved parties is required to provide a knowledge-based approach, in-depth risk understanding, and sustainable mitigation measures. By acceptance of the transfer plan and initiation of change control, the planning phase has been completed.
The tech transfer execution usually starts with the transfer of analytical methods, including training of operators and qualification of assays. In parallel, the manufacturing process transfer occurs. Driven out of a facility fit assessment, necessary plant modifications are performed and process adaptations (e.g., scale up, volume adaptations, operational parameter verification) are performed. These are typically determined out of a sitespecific process description owned by the receiving site and verified by the donating site. The risk management process plays an important role, since validation and qualification documents, operating procedures, and process adaptations are designed as a result of the risk assessments. In addition, the risk assessment enables a structured analysis of impacts to other products produced in a multiproduct facility. Alignment and establishment of tech transfer success criteria are vital, with data management and statistical competencies as key enablers. One example is the definition of the 95/99 tolerance intervals (TI) based on data from historical batches. Often, the establishment of primary and secondary success criteria drives aspects of the process comparability exercise and enables a strong framework of decision making. For example, the "Certificate of Analysis" criteria might be a primary criteria; however, in order to assess process trends or compare the results to a historical database of the manufacturing process, secondary criteria should be established, including the historical ranges (which are often tighter criteria). Consistency of the manufacturing process (as executed at the donor site) should also be analyzed and described in the validation package.
After the implementation of all necessary documents, scale-down model qualification of potential process changes, and qualification of equipment modifications, the verification of at-scale performance is made by engineering runs or technical runs. The engineering runs are typically the “rehearsal” of all systems, processes, and people required to manufacture the transferred process. In order to allow the buildup of experience and knowledge, training of receiving-site operators at the donating site might be required. It is recommended to apply defined success criteria as a gate for proceeding into qualification runs. These allow for a clear decision and a more structured approach for discussion and potential troubleshooting. In order to enable correction of potential issues during engineering runs, a low run rate and sufficient time between batches should be planned. If all criteria are met, the execution of the qualification runs is the next step.

Figure 4. ICH Q9 risk management process
The number of qualification runs depends on several factors, including regulatory requirements/strategy and assumed successccess is critical. The qualification runs need to be repre rates at the receiving manufacturing site, which are driven by risk assessment and experience. The qualification runs need to be monitored carefully, since their susentative and meet the pre-defined acceptance criteria. In addition, a pre-defined set of consecutive qualification runs needs to be completed and submitted to the agencies with the filings. During the qualification runs, the process monitoring protocol (including control limits) will be determined as well. Finally, the validation protocols, comparability reports, and other essential documentation will be prepared. In some cases, a feedback loop to the risk assessments might be beneficial. Upon execution of the transfer, the registration phase starts with the preparation of the dossier. An equivalent body for review might be different to the steering committee. In most cases, a preapproval inspection by the regulatory authorities might be necessary – distribution of produced material is only possible after approval by the authorities, while production might occur before to support potential ramp up plans, stock buildup, and supply chain strategies. The success of transfers depends on four pillars:
- Clear business processes including milestones and deliverables
- Appropriate governance structure with a team of knowledgeable people with clear roles and responsibilities. Availability of appropriate resources is essential.
- Process and equipment knowledge (incl. knowledge management)
- Communication and decision making Risk Management Aspects
Risk Management Aspects
Pharmaceutical companies deal with general and product/processspecific risks. The ICH Q9 is a high-level model for Risk Management adopted by the FDA in June 2006, as well as EU Annex 20 in February 2008. Each of these tools has advantages and disadvantages, which will not be explained in this article. Figure 4 shows the general steps in the risk management process as outlined in ICH Q9. Quality risk management is a systematic process: objective, transpevarent, data-driven, multidisciplinary, focused on product quality and patient safety, and applicable throughout all phases of the product lifecycle, therefore, an essential part of technology transfers. A structured approach, the use of adequate tools and communication, and documentation are key. Risk management is ongoing and not a one-time exercise. Assessment, control and ongoing review are essential elements of the process.
During tech transfer, adaptation of processes and equipment needs to be performed. The identification of hazards and risks early in the transfer process allows timely mitigation as well as a structured way to design validation test protocols, validation attempts, and comparability protocols in alignment with regulatory expectations.
As outlined in Figure 4, the quality risk management starts with the risk assessment phase, including: identification, analysis and evaluation. In most cases, manufacturing scale changes exist between sites and scale-up has to be performed. A common risk in cell culture is that of adequate mass transfer and mixing, for example. A suitable scale-up strategy is based on process knowledge and equipment knowledge, including demonstration of this parameter in experiments to determine the so-called “window of operation”. Figure 5 illustrates the activities for risk management during the transfer of a cell culture process, including a 5-fold scale up from 5,000 to 25,000 L.
As can be concluded from this short example, the relevance of a structured end-to-end approach is obvious. In order to enable solid assessments of these changes and to provide adequate regulatory strategies and scientific assessments, knowledge for equipment and processes is absolutely required.
Equipment Characterization and Process Knowledge
Along with a strong foundation comprised of governance structure, risk management and regulatory expectations, the key for a successful transfer is knowledge around process, equipment and quality assays to demonstrate product and process comparability for commercial manufacturing. The use of simulation tools and scientific models, subject matter expertise, data and knowledge management are key to avoiding pitfalls and accelerating timelines. The following text will give examples and illustrate this in several short case studies.

Figure 5. Risk management example for the transfer and scale up of a cell culture production process
- Equipment Knowledge and Facility Fit - Differences in facilities and equipment typically exist between donor and receiving sites, resulting in scale differences, disparate vessel and tank volumes, and other detailed equipment differences (impeller types, sparger, baffles, etc.). At the start of the execution stage, a facility fit assessment of the receiving site capabilities is performed to verify process equipment and control systems in alignment with the process requirements. This detailed analysis of facility and equipment capabilities is required to allow science- and risk-driven process adaptation, while minimizing process changes as to avoid impact on process performance and product quality. Although a high level gap assessment is typically executed during the initial due diligence visit, the intent of this detailed facility fit assessment is to evaluate every processing step and equipment in greater detail. Considerations for the facility fit assessment include the following:
- Sizing of equipment and identification of bottlenecks – identification of long lead items for potential facility modifications
- Infrastructure and system capabilities
- Quality systems
- Laboratories and analytical equipment
Operating parameters and analytical equipment can have tremendous impacts on performance of cell culture-based manufacturing processes. Detailed characterization of key unit operations and their impact on culture process performance needs to be conducted, especially if the transfer involves non platform processes, scale differences, etc. For cell culture processes, special emphasis should be drawn to physical characteristics of bioreactors. In general, these have to provide sufficient mixing, mass and heat transfer at low shear forces:
- Mass transfer for O2 and CO2
- Mixing characteristics, including requirements for macro- and micro- mixing
- Shear force by power input (stirring, gas throughput)
Structured scale up and transfer rules should be applied based on the process requirements and equipment characteristics. Common scale-up bases include power input per volume, tip speed, and superficial gas velocity constant. However, keeping one basis constant requires understanding variability in the others. Since knowledge of the unit operations described above is absolutely necessary (including the conclusion of dimensionless relations), a series of experiments is required to minimize impact on cell culture performance and product quality attributes. To avoid utilization of costly large-scale capacity model system, simulation tools are used in the industry to enable “frontloading” and risk assessment at an early point in the transfer. Popular methods include computational fluid dynamics (CFD), capacity utilization models, experimental model reactors (e.g. PVC vessels) and scale down models, to determine/approximate mixing times, mass transfer, shear force, and facility bottlenecks without occupying costly time in the operational equipment. Next to the equipment, special attention should be drawn to validation and comparability of analytical assays, procedures and equipment. A detailed analysis is required since minor differences can have a tremendous impact on results, for example, “trivial” off-line pH measurement or cell count devices on the floor, or method differences and upgrades in quality control laboratories. The following case studies illustrate these points.
Case study 1: Simulation and Determination of Window of Operations for a Large-Scale Cell Culture Process

Figure 6. Example of a mixing profile evaluation of a 16,500L bioreactor at low working volume
An example of a typical scale-up approach involved a transfer of an existing approved product, where a scale-up from the 12,000-L cell culture manufacturing scale to 16,500-L manufacturing scale with a corresponding increase in the scale of the inoculum train bioreactors occurred. Each bioreactor in the inoculum train and production was analyzed, and the scale-up appropriately conducted to ensure harmonization with existing manufacturing facilities and process performance within expected ranges. The sparge flow rate and the overlay gas flow rate were scaled up to give equivalent volumetric flow rates (vvm) as defined per unit liquid working volume as the scaling parameter. The agitation rate was scaled up to give equivalent specific power dissipation rate per unit liquid working volume as the appropriate scaling parameter. The risk assessment showed no significant risks for the seed train.
Moreover, for the production scale, detailed geometry comparison and set up showed significant differences, which were assessed in a risk management process. The risk assessment showed a potential impact to cell culture due to a non-fitting split ratio of the working volume in the pre-production (for expansion of cell culture designated as N-1) and production (N) cultures, as well as concerns for the critical unit operations in terms of mixing, mass transferand shear force.
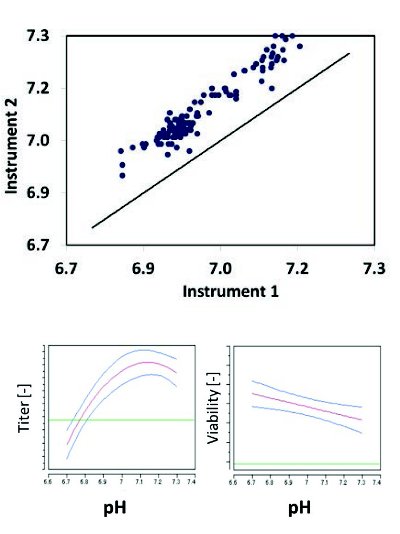
Figure 7. pH offset is observed between two different pH offline instrumentation led to a shift in the process operating target at manufacturing scale. Regression model from small scale characterization studies showed pH influence to cell culture production performance.
The volume for the pre-production culture was atypically low and a risk for mixing and aeration was identified using a quality risk management tool. A process adaptation leading to an increase of the split ratio was not possible due to media design, “overfill” of the production bioreactor, missing process validation work, etc. An investment into a different vessel was not possible due to facility limitations. It was decided to maintain the split ratio equivalent to the transferring site and perform detailed physical characterization for the low volume in the existing tank (approximately 25% of the nominal vessel volume). In addition, scale down cultivations were performed to assess the impact to cell culture performance and product quality. The following points give an overview of the performed work and analysis in both experimental bioreactors at 1000 L and 400 L scale, as well as CFD simulation, and a small number of experiments at scale to verify assumption (see Figure 6).
- Shear stress analysis – a concern was raised on impact of hydrodynamic shear damage of cells in the impeller zone at low working volume. An established database of bioreactor characterization across the manufacturing network was utilized to evaluate the typical P/V range utilized at expansion stage for mammalian cell culture. This target P/V range was utilized as baseline to determine capability of the bioreactor to maintain the target P/V range at this low volume and target agitation rate. Moreover, liquid level at ½ of the impeller diameter above the impeller was utilized to avoid bubble entrainment or cavities creating shear force. Here, CFD simulations and a model particle system (blue clay polymer system) were used.
- Mixing and mass transfer analysis –Mixing studies were executed to address these factors using different methods (conductivity change, de-colorization method) and corresponding CFD models for several power inputs. Furthermore, mass transfer experiments for different power inputs were conducted in model bioreactors, simulated in CFD models to drive in-depth understanding. Finally, a limited number of verifying experiments were performed at scale.
- The operational parameters were concluded and cell culture performance was tested at small scale (2 L and 10 L) to verify the impact of the determined operating condition.
As a result of these analyses, the risk was assessed and controls put in place according to the quality risk management process leading to an acceptable level of risk. The engineering and qualification runs were successfully conducted with the determined operational parameters. This approach led to mitigation of risk, avoiding expensive time at scale to optimize the process, and knowledgeable decision-making.

Figure 8. The role of scale down models in transfers
Case Study 2: Difference in pH Measurement for Production Culture
A commercial monoclonal antibody manufacturing process was transferred between two sites within the global manufacturing network. Lower cell culture performance and product titers were observed at the receiving site when compared to the donating site at small-scale experiments. A root cause analysis and risk assessment showed nontrivial differences in off-line pH measurements, due to instrumentation and procedural differences (e.g., blood gas analyzer versus bench top pH meter). The pH offset between the two offline instruments corresponded to a shift in the process operating target when offline pH was used as point of reference for calibrations. The extent of the impact of pH offset in the cell culture process was further confirmed with a regression model that showed influence of pH on cell culture growth and yield performance utilizing small scale studies. The subsequent adjustment at manufacturing scale before engineering and qualification runs led to comparable performance and product titers. This case study illustrates the importance of detailed understanding of differences among various methodologies, and such artifacts must be well understood to ensure that a process operating condition remains the same upon transfer to a new manufacturing site. Along with process characterization and understanding, the ability to recognize and correct this issue can be facilitated utilizing small scale-models and structured troubleshooting, as well as risk management processes.
- Process Knowledge – process characterization, process validation, ongoing process monitoring including statistical process control, strengthening the knowledge-base, and enabling identification of improvement opportunities during lifecycle of a process. A knowledge-based approach enables better risk management, decision making and trouble shooting, as well as assessment of potential process changes to ensure comparability during technology transfer. This knowledge serves as the framework for determining the process operating window, as well as success criteria for process validation and comparability protocols during tech transfer. The determination of the process operating conditions or operating window is an essential aspect when analyzing the receiving site’s process equipment and control systems capabilities to be in alignment with process requirements. Next to the previously-mentioned physical characterization, predictive scale-down models play an important role in assessing reproducibility of manufacturing scale performance upfront, and therefore, will mitigate risk. Scale-down models are essential from process development through execution of product transfer process. Where replicates cannot be run at manufacturing scale, scale-down models play an important role in process design, process characterization and validation, and troubleshooting to support manufacturing throughout the product lifecycle. Understanding the performance of the scale-down model at the receiving site is essential to having a meaningful assessment on its ability to predict performance at manufacturing scale and identifying risks early in the process without consuming a large amount of resources. For cell culture model qualification (typically, laboratory scale) operated at normal operating targets as manufacturing scale, comparability is typically based on cell culture key process indicators, product quality attributes, and impurities that are relevant to the unit operation. During a transfer, product quality assays and scale-down models are transferred first, since they serve as a comparison of operations at equal scale to identify potential systemic differences and offer an opportunity for training of staff. They also serve as a central tool for continuous improvement of the process during the lifecycle.
Risk Mitigation and Continuous Improvement during Technology Transfers by Implementation of Mature Technologies Post-Approval
Increasing robustness, “upgrading” control strategies, mitigating risks and increasing yields without compromising product quality and consistency of processes is essential during the product and process lifecycle. From target identification to market, the commercial product lifecycle encompasses several stages and focus on these improvements may vary throughout the stages of these product lifecycles. Although, being strictly regulated post approval is required in in alignment with expectations of the regulatory authorities to guarantee a reliable supply of high quality products. The implementation of new process versions driven out of process development, adaptation of control strategies or the implementation of mature “state-of-the-art” technologies to improve commercial manufacturing processes is an opportunity during tech transfers. However, a suitable implementation strategy, regulatory requirements, risk management for the implementation, as well as business and supply chain aspects have to be considered. Knowledge management during the lifecycle is therefore critical to identify and assess potential improvements and implement a suitable regulatory strategy. If managed successfully, the realization of these improvements will lead to more robustness, efficiency, and agility in manufacturing processes throughout the network, contributing to a competitive edge of the company.
Scale-down models play a central role in assessing the risks of implementing mature technologies and continuous improvement purposes (e.g. disposable applications, high temperature short time treatments, etc.). It is important to connect equipment and process knowledge with a stringent, quality risk-management approach. Experience from comparable products within the company, scientific peer reviews, and other sources need to be considered. The following case study provides an example of these aspects.
Case Study 3: Implementing Mature Technologies During Tech Transfer by Applying Knowledge Management and Scale-Down Models
To increase manufacturing capacity and ensure continuous supply of commercial material, the approved process was transferred to a multiproduct manufacturing facility in the existing network. Because of the complexities of large-scale processing and the variety of possible entry points for adventitious agents, multiple barriers have been designed and implemented, including heat treatment of animal-derived raw materials as one additional barrier to mitigate supply chain risk. High temperature short time (HTST) treatment of large volume cell culture media is a mature technology which has been implemented in several manufacturing processes. Within the company, tremendous knowledge and experience of applying HTST to cell culture media exists. In order to assess the risk of implementing this technology during transfer in the existing manufacturing process, a stringent risk management process was applied involving several SMEs assessing potential risks for cell culture performance, process consistency and product quality. Driven out of the risk assessment, targeted test protocols and studies were designed. Initial test runs showed media component precipitation in small scale and pilot scale when applying HTST, leading to filter clogging and cell culture performance impact. Detailed root cause analysis and continuous risk management led to procedural adjustments to the media preparation process, while no modification to media compositions was performed. Driven out of the risk assessment studies to assess both, the impact to HTST treatment and subsequent cell culture performance, product quality and process consistency were tested in numerous small scale studies. After reviewing the positive results at small scale matching the success criteria derived from historical at scale data, it was decided to implement the mature technology at scale during engineering runs and qualification runs. Distinct success criteria for engineering runs and qualification runs were concluded and adequate validation protocols were designed. As demonstrated in small scale, the procedural adjustments and the use of HTST heat treatment did not compromise the quality of the cell culture as confirmed by the comparability assessment during the licensure campaign. The options for HTST heat treatment of media were successfully implemented as a business risk mitigation option for the process under a Post Approval Supplement for licensure of the additional facility. Exemplary results for cell culture performance and at full scale are shown in Figure 9.
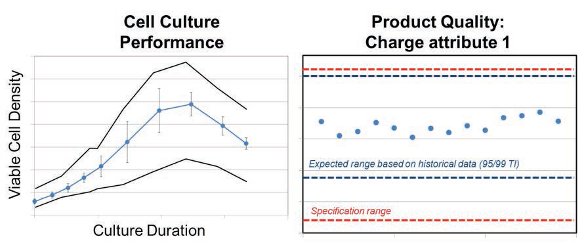
Figure 9. Exemplary at scale result from Engineering and Qualification runs
Conclusions
In summary, this article provides a holistic view of technology transfers from end to end. A clear and aligned business process with clear roles and responsibilities is required to avoid disconnects. Process planning and execution, clear governance structures, good communication, and collaboration between subject matter experts from different functions around the world are basic principles for ensuring success and good decision making. Quality risk management and a suitable regulatory strategy driven out of regulatory guidelines, in combination with excellence in knowledge management, are key for risk mitigation activities and sufficient control. Tools such as CFD models and capacity simulation (in conjunction with scale-down models) will enable mitigation and understanding of risks upfront to avoid costly pitfalls at large scale. The combination and stringent management of these aspects and principles, in alignment with regulatory expectation, will mitigate risk, optimize performance and enable successful tech transfers.
References
- Reichert JM. Monoclonal antibodies in the clinic. Nat Biotechnol. 2001; 19:819–822.
- Reichert JM. Therapeutic monoclonal antibodies. Trends in development and approval in the US. Curr Opin Mol Ther. 2002; 4:110–118.
- Reichert JM, Pavolu A. Monoclonal antibodies market. Nat Rev Drug Discov. 2004; 3:383–384.
- Drapeau M, Sullivan, F, Moniz Carpenter, J. Special Report: Blockbuster Then and Now-Trends for Billion-Dollar Drugs. Spectrum Therapy Markets and Emerging Technologies. 2007; 12:1–39.
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nature Reviews. 2009; 8:959–968.
- Xie L. Wang DIC. Integrated approaches to the design of media and feeding strategies for fed batch cultures of animal cells. Trends Biotechnol. 1997; 15(3):109–13.
- Qi HN, Goudar CT, Michaels JD, Henzler HJ, Jovanovic GN, Konstantinov KB. Experimental and theoretical analysis of tubular membrane. Biotechnol Prog. 2003; 19(4):1183–1189.
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004; 22:1393–1398.
- Sethuramann N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol. 2006; 17(4):341– 346.
- Heath C, Kiss R. Cell Culture Process Development: Advances in Process Engineering. Biotechnol Prog. 2007; 23:46–51.
- Su WW. Bioreactors, Perfusion. Encyclopedia of Cell technology. 2003; 978–993. John Wiley & Sons. Inc.
- Shukla A, Thömmes J. Recent Advances in Large-Scale Production of Monoclonal Antibodies and Related Proteins. Trends in Biotechnol. 2010; 28:253–261.
- Chang D. “Biologics Product & Process Lifecycle Management: Genentech Roche Experience” IBC Conference San Diego, February 2012
Michael Pohlscheidt received his degree in bioengineering in 2001 from the University of Applied Sciences in Aachen, Germany. His Ph.D. thesis was performed at Bayer HealthCare AG and Bayer Technology Services and guided by the University of Magdeburg, Germany (2005). From 2005 to 2010, he worked in different positions at Pharma Biotech Production & Development, Roche Diagnostics GmbH, Penzberg, Germany. Since 2010, he has worked at Genentech Inc., Oceanside, CA, first as an associate Director Manufacturing Science and Technology and since 2012 as Director of Manufacturing Operations.