Introduction
Packaged pharmaceutical drug products can interact with their packaging, resulting in the migration of substances from the packaging and into the drug product. The presence of migratory substances in the finished drug product is of concern due to the impact that such substances could have on the finished drug product’s suitability for use. Thus it is necessary and mandatory that the finished drug product’s manufacturer ascertain the extent of migration and establish that the migration substances’ impact is within acceptable limits. Doing so requires that the migrating substances be discovered, identified, and quantified in a finished drug product via a process termed chemical assessment.
Because the development of a finished drug product is a long and involved process, chemical assessment is typically a series of actions which together represent a logical and efficient process of risk management. This series of actions has been captured in the Chemical Assessment Triad (Figure 1, [1]), which establishes the three essential stages of chemical assessment; material screening and selection, the controlled extraction (simulation) study and the product assessment (migration) study.
Use of Material Characterization Data for Material Screening and Selection
During product development, candidate materials for use in the packaging system are identified and either chosen for use or rejected based on quality and performance attributes such as their composition as well as their mechanical and chemical properties. One important expectation is that the material not contain potentially unsafe or reactive ingredients, additives or processing aids. During the selection process, candidate materials are characterized to establish their composition (i.e., identity and quantity of their ingredients). These ingredients are assessed, in a generally qualitative manner, to establish the extent to which they could lead to extractables and leachables. Inappropriate materials (those which have ingredients that could produce undesirable extractables or leachables) are eliminated from further consideration, increasing the likelihood that the finished drug product will be unaffected by its packaging. Thus, material characterization achieves the dual objectives of proper risk management and appropriate quality design.

Figure 1. The Chemical Assessment Triad. Executing the processes of Material Screening and Selection, Controlled Extraction Simulation Study and Product Assessment throughout the product development process effectively manages the risk associated with substances which migrate from packaging and into a drug product. From reference 1.
The proposal that ingredient data could be an effective screening tool for candidate materials of construction is based on the concept that there is some relationship between ingredients in materials, extractables in packaging systems and leachables in packaged products (for example, Figure 2 and [2-5]). Such a concept is acceptable for material screening as it is recognized that the screening process is a risk mitigation tool and not a formal risk assessment tool. More specifically, while materials data may be used to select materials, it is rarely sufficient, in and of itself, to establish the safety of, and to secure regulatory approval of, packaged drug products. Thus the relationship between ingredients, extractables and leachables does not have to be quantitative, or even well-defined for materials screening based on ingredients, in order to be effective. Should the material screening be faulty (that is the packaging system contains a material which is the source of an undesirable leachable), such an outcome will be surfaced in either the subsequent extractables or leachables studies. Therefore, there is neither the intent, nor the requirement, that material screening be sufficiently comprehensive that all potentially inappropriate materials are screened out; rather, material screening should a way to reveal and avoid those materials that are poorly suited for a particular application.
Use of Material Characterization Data to Facilitate Extractables and Leachables Profiling
The process of specifying and quantifying extractables in a packaging system extract or leachables in a packaged drug product is an exercise in analytical profiling wherein an extract or drug product is chemically characterized and an extractables or leachables profile, consisting of a list of compounds and their concentrations, is generated. Under any circumstances, the analytical profiling process is challenging and resource-intensive; however, the degree of difficulty increases in indirect proportion to the amount of information that is available about the test system and its composition. When little or nothing is known about the test system prior to profiling, the analytical chemist must profile a test article which is essentially a black box. In such a situation, the analyst has no basis for setting expectations and thus is essentially “flying blind”. Because nothing is known about the composition of the test article, the screening methods used to establish the extractables or leachables profile must be sufficiently robust and broad scope to capture the entire diverse universe of potential extractables. In the absence of compositional information, screening methods cannot be optimized so that the methods work particularly well for “known” extractables. Similarly, the screening methods must be highly sensitive and possess a wide dynamic response range, as the extractables (or leachables) may be present in the extracts (or drug products) at concentrations that are low enough to challenge the method’s detection capability or high enough to challenge the method’s dynamic range. Lastly, because all responses obtained by the screening methods are unanticipated, the investigation of these responses to procure the extractables’ identity and concentration is prolonged and rigorous.
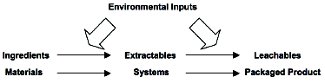
Figure 2. The Evolution of Leachables from Ingredients and through Extractables. Ingredients that are present in a system’s component materials are a logical source of extractables. These ingredients can be extractables themselves or they might contain impurities or produce decomposition products which may also be extractables. Furthermore, extractables may be derived from chemicals used to process materials into components or systems. Similarly, extractables are the logical source of leachables. The leachables may be the extractables themselves or they may be substances derived from extractables due to their decomposition or reaction. Environmental inputs such as manufacturing, sterilization, storage, shipping and use may cause the ingredients to be converted into extractables (or extractables into leachables) or may add substances (which are not ingredients) to the population of extractables (or leachables).
This potentially inefficient situation can be contrasted to an alternate case wherein the test article’s composition (ingredients profile) is known. It is the current state of the science that the chemical behavior of many of the more commonly utilized ingredients is well-known and well-documented. In cases where such documentation is scarce, chemical behavior can be inferred by a number of appropriate and effective methodologies. In either circumstance, a review of a particular ingredient can produce a list of potentially related substances (extractables).
The mechanical and physical properties of the materials can also be used to forecast the extent to which its ingredients will migrate. Traditional materials, like metal and glass, have superior barrier properties compared to polymers and are generally more resistant to leachables. Polymers offer more versatility than metal or glass, since they are lightweight, flexible and much more durable than their traditional counterparts. Polymers can simultaneously show properties of crystalline solids as well as viscous liquids. The terms crystalline and amorphous are used to describe the ordered and unordered regions in a polymer system. The degree of crystallinity can vary by polymer type and will govern many of its overall properties. If a polymer chain has structural regularity, is compact, and has some degree of flexibility, it will be more susceptible to taking on a crystalline form because its chains will be conducive to packing into organized morphologies. The degree of secondary intermolecular forces, such as those from polar side groups or hydrogen bonding, will also cause a polymer to assume a more crystalline morphology. Typically, the more highly ordered polymers have a greater degree of crystallinity, are more rigid, and less prone to leachables. Examples of these polymers include polystyrene and polycarbonate. The softer, amorphous polymers are generally more susceptible to leachables. Low density polyethylene is an example of an amorphous polymer while polypropylene and high density polyethylene are types of semi crystalline plastics. Migration is more likely to occur in amorphous polymers and in semi crystalline polymers at temperatures above their glass transition temperatures.
The extent to which leachables could migrate from materials and into the drug or drug components depends on several key factors:
- The type of material used to construct the equipment in question
- The compatibility of its ingredients or additives
- The solvating power of the drug with respect to the material (pH, polarity, and ionic strength)
- The temperature
- The duration of contact
- The surface area to volume ratio
A list of potential extractables for a packaging system would consist of the combined lists of ingredients and their related substances for the system’s materials of construction. Furthermore, the chemical properties of the ingredients and related extractables (for example, their octanol/ water partition coefficients and acid- or base-dissociation constants), in conjunction with their total pool in the test material, could be used to estimate (or forecast) the accumulation levels of the individual substances as extractables or leachables. In essence, such an assessment, based solely on knowing a test materials composition, produces a forecasted extractables (or leachables) profile. Armed with, and guided by, such a forecast, the analytical chemist is well-positioned to perform an effective and efficient extractables (or leachables) screening study.
Case Studies: Correlating Ingredients, Extractables and Leachables for Three Materials Used in Parenteral Product Packaging
Currently, an Extractables and Leachables Working Group within Product Quality Research Institute (PQRI) is extending best demonstrated practice recommendations for extractables and leachables safety assessment developed for orally inhaled and nasal drug products (OINDP) to other dosage forms, including parenterals and ophthalmics (PODP) [5]. To this end, the PODP’s Chemistry team has initiated a series of laboratory studies to produce the data and information needed to develop and support best practice recommendations. The first study, consistent with the concepts of the Chemical Assessment Triad, were controlled extraction studies performed on materials that are commonly used in packaging associated with the PODP dosage forms [6]. As the compositions of the test materials were specified prior to their characterization, the PODP study serves as an appropriate case study to illustrate the concept and utility of the ingredient-extractables correlation. Such a correlation will be illustrated for three of the materials included in the PQRI study: a DEHPplasticized poly vinyl chloride (PVC), a low density polyethylene (LDPE) and a halobutyl rubber. A consideration of the DEHP-plasticized PVC case was first reported in reference 7.
DEHP-plasticized PVC
The composition of the DEHP-plasticized PVC test article is summarized in Figure 3. Among its formulation components are the PVC resin itself, DEHP as the primary plasticizer, epoxidized oil as the secondary plasticizer, metal stearate salts as acid scavengers and erucamide as a slip agent (among other potential functions). Although the test article itself is not used in commercial applications, its composition is consistent with plasticized PVC materials that are used in pharmaceutical applications.
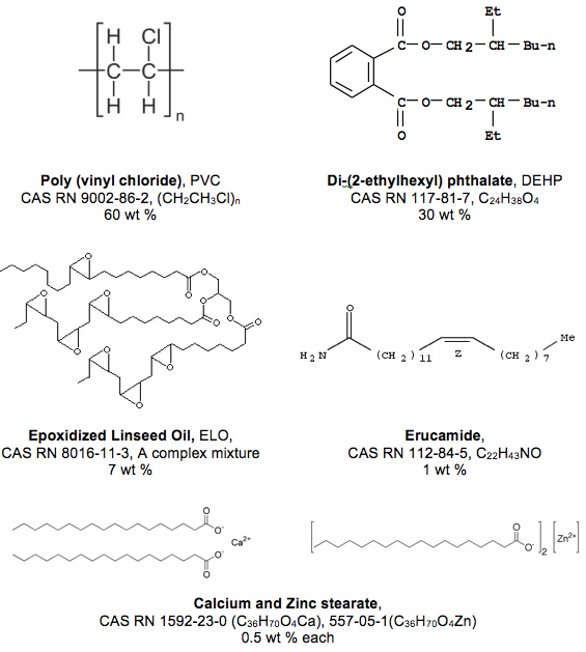
Figure 3. The Ingredients in the DEHP-plasticized PVC Test Material.
Figures 4 through 8 illustrate the related substances profiles of the individual PVC components. As plasticized PVC materials have been characterized extensively for extractables and leachables, significant quantities of information about related substances is readily available and obtainable in the chemical literature. For example, the potentially extractable substances that are typically related or attributed to the PVC resin itself include the PVC monomer and hydrochloric and acetic acids (Figure 4). A more extensive set of related substances can be generated for the other PVC components. The Figures present within this manuscript contain only the more commonly reported or more easily envisioned and justified related substances.

Figure 4. PVC Extractables Associated with the PVC Base Resin.
Although generating a list of potential extractables from the compositional information is a useful endeavor, an even more useful exercise would be to forecast the semi-quantitative nature of an extractables profile that would be obtained under specified extraction conditions. To accomplish this objective, several pieces of information are relevant; for example, properties of the extractable that establish their thermodynamic behavior, properties of the extractables that establish their kinetic behavior, properties of the test material that affect the migration of compounds through the material and the total pool of the extractable in the test material. When the extraction is performed under conditions which achieve equilibrium between the test material and the extracting medium (asymptotic extraction), the kinetic factors become irrelevant.
Table 1. Forecasted Organic Extractables Profile for the DEHP-plasticized PVC

While quantitative extractables profiles can be mathematically generated if such quantities as total pool, partitioning behavior, and extraction stoichiometry (amount of material extracted per unit volume of extraction solutions) are known, qualitative profiles can be established with less rigorous data and by less rigorous methods. Such a forecasted extractables profile is contained in Table 1. A qualitative line-by-line justification of the entries in this Table is as follows:
Vinyl chloride monomer. Vinyl chloride monomer is rarely present in significant quantities in modern PVC materials, meaning that the available pool of this potential extractable is low.
- Hydrochloric and acetic acids. These highly soluble low molecular weight acids are present in PVC materials in low quantities, unless the material is gamma irradiated.
- DEHP. The large pool of this ingredient contrasts with its limited aqueous solubility (log P > 8.5). The molecule is not ionizable and thus its accumulation in aqueous extracts will be low and pH-independent. Given its large pool, DEHP could accumulate in organic solvent extracts in higher quantities.
- MEHP. If MEHP is present in the DEHP ingredient as an impurity, its levels are typically low. Thus its levels in an organic solvent extract would be low, reflecting the low pool. Considering aqueous extracts, it is noted that MEHP solubility increases with increasing pH and furthermore that the hydrolysis of DEHP to form MEHP is base-catalyzed. Thus MEHP levels in aqueous extracts will increase with increasing pH of the extracting solution.
- 2-Ethyl-1-hexanol. 2-Ethyl-1-hexanol, a DEHP degradation product, has a moderate total pool in DEHP-plasticized PVC materials. This molecule is not ionized and thus its accumulation in aqueous extracts is not pH-dependent. It is moderately soluble in aqueous media and thus it is anticipated that its levels will be somewhat higher in an organic, versus an aqueous, extract.
- Phthalic acid. This is a similar discussion to MEHP although phthalic acid more typically is an impurity in the DEHP and is not generated hydrolytically by the extraction medium.
- Dibutyl phthalate. This compound is present in the plasticized PVC in very low quantities. Given its low water solubility, it will not accumulate in detectable quantities in the aqueous extracts. Based on its small pool, its levels in organic extracts would typically be lower than the detection limit.
- 1(3H)-Isobenzofuranone. This compound is a very minor extractable in terms of total pool, and is only rarely reported as an observed extractable.
- Epoxidized fatty acids. This class of compounds has a fairly large total pool and, as acids, can accumulate at detectable levels at higher pH values due to their increased solubility. As uncharged molecules in an organic extract, the epoxidized fatty acids will accumulate to levels dictated by their individual octanol-water partition coefficients and the polarity of the organic extracting solution.
- Expoxidized fatty acid esters. As esters, these extractables will not have the pH-dependent solubility of the acids and thus will not accumulate in aqueous extracts because of their low aqueous solubility. In organic extracting solvents, the esters will accumulate to levels dictated by their individual octanol-water partition coefficients and the polarity of the organic extracting solution.
- Fatty acids. The fatty acids will behave in the same manner as the epoxidized fatty acids with respect the extracting solution type. The total pool of fatty acids may differ from the total pool of the epoxidized fatty acids so the levels of these two groups may be different.
- Erucamide and other amides. These are low aqueous solubility compounds whose solubilities are not pH-dependent. Thus they will not accumulate in aqueous extracts at detectable levels. Because their pool in the material is moderate, they will accumulate in organic extracts to levels dictated by their individual octanol-water partition coefficients and the polarity of the organic extracting solution.
Table 2. Organic Extractables Profile of the PVC Material; Identified Extractables at Levels of 1 μg/g or greater.

Proof of concept is accomplished when the forecast contained in Table 1 is compared with the results of actual extraction studies. As noted previously, this test material was characterized for its extractables profile through the efforts of the PQRI PODP Extractables and Leachables Working Group [6]. This characterization involved extraction of the test materials by several means, including:
- Sealed vessel extraction with aqueous solutions at high and low pH and with a 1/1 (v/v) mixture of ispopropyl alcohol (IPA) and water, and
- Reflux and Soxhlet extraction with IPA and hexane.
The resultant extracts were chromatographically characterized for organic extractables; the resulting extractables profiles are summarized in Table 2. In comparing Tables 1 and 2, the following is noted:
- Most of the identified extractables in Table 2 were forecasted in Table 1, and
- In general the qualitative concentration trends forecasted in Table 1 are consistent with the semi-quantitative data provided in Table 2.
Furthermore it is noted that most of the extractables listed in Table 2, which were not directly forecast by Table 1, can logically be linked to the ingredients of the test article. Therefore it is logical to anticipate that, if the individual fatty acids are both forecast and measured extractables, their methyl esters would also be present in the extracts in measurable quantities. Similarly, the presence of fatty acids and fatty acids esters other than those directly forecast in Table 1 in the extracts is logical given the composition of the test article (fatty acid salts and epoxidized oil). Armed with the ingredient-extractables correlation, the next challenge is to finish the chain and add leachables to the correlation. As the accumulation of leachables is situation-specific, such a correlation can only be built for a particular product type (or product category). For the purpose of this case study, a product category is created as follows:
- Aqueous drug product containing highly water soluble active pharmaceutical ingredients (API), formulated in commonly utilized diluents (e.g., Normal Saline, 5% Dextrose)
- These aqueous drug products are formulated in a pH range of 3 to 9.
- These aqueous drug products are not formulated to contain additives or co-solvents whose purpose is to increase the solubility of the API.
- The aqueous drug products are filled into properly-sized containers, whose sole primary material of construction is the specific PVC discussed previously in this document.
- The fill volumes of the packaged drugs products range from 50 to 1000 mL.
- Containers filled with the drug product are terminally sterilized.
- The shelf-life of the product is 24 months at a nominal temperature of 25°C.
- Under these circumstances, the extractables information obtained with the aqueous extraction solvents is most relevant and it is concluded that the drug product and its container will reach equilibrium at some time during its shelf-life. Thus the forecasted level of leachables in such a drug product will be based on thermodynamic, as opposed to kinetic, modeling.
Table 3. Organic Leachables for Aqueous Drug Products in DEHP-plasticized PVC Containers
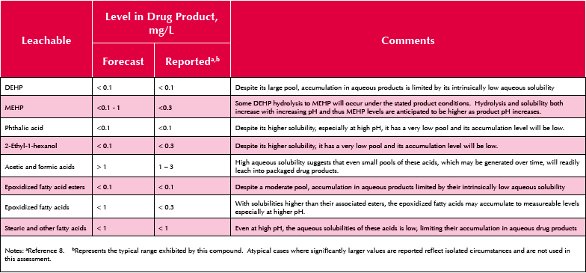
Such a forecasted leachables profile is contained in Table 3. The forecasted profile is compared with leachables data that has been reported for drug products that meet the general description provided previously. A qualitative line-by-line justification of the entries in this Table is as follows:
- DEHP. The large pool of this ingredient notwithstanding, DEHP will accumulate as a leachable to levels no higher than its intrinsic aqueous solubility, which is on the order of 0.1 ppm.
- MEHP. The small intrinsic pool of this compound will mean that its accumulation in solution will be limited, except at higher pH where DEHP hydrolysis might produce MEHP levels that approach 1 mg/L.
- Phthalic acid. The very low pool of this extractable will limit its accumulation as a leachable to levels below 0.1 mg/L.
- 2-Ethyl-1-hexanol. Despite its relatively high water solubility, its pool as an extractable is low and this substance will accumulate as a leachable to levels near the 0.1 mg/L level.
- Acetic and Formic acids. These highly water soluble acids will accumulate as leachables to the full extent of their total available pool, which is estimated to produce leachables levels in excess of 1 mg/L.
- Epoxidized fatty acids. This class of compounds has a fairly large total pool and as acids can accumulate at detectable levels at higher pH values due to their increased solubility. However, even at high pH, their aqueous solubility is limited and their accumulation levels as leachables will typically be less than 1 mg/L.
- Expoxidized fatty acid esters. As esters, these extractables are only poorly soluble and will not accumulate in as leachables in aqueous drug products in levels much above 0.1 mg/L.
- Fatty acids. The fatty acids will behave in the same manner as the epoxidized fatty acids with respect to their behavior as leachables in aqueous drug products.
As noted in Table 3, the forecasted accumulation levels are in line with the range of leachable concentrations that have been reported. Given the complexity of drawing quantitative correlations between ingredients, extractables and leachables, the qualitative agreement between forecasted and observed leachables levels reflects a reasonable extrapolation of the existing information. While one could envision situations in which the available data might provide the basis for more semi-quantitative leachables forecasting, this was not the case for the data that is the basis of this case study.
References
- Jenke, D. A general strategy for the chemical aspects of the safety assessment of extractables and leachables in pharmaceutical drug products: The chemical assessment Triad. PDA J Pharm Sci Tech. 66(2): 168-183 (2012).
- Jenke D. Linking extractables and leachables in container/closure applications. PDA J Pharm Sci Tech. 59(4): 265-281 (2005).
- Ruberto, M.A.; Paskiet, D.; Miller, K. Chemical and physical attributes of plastics and elastomers: Impact on the extractables profile of container closure systems. In Leachables and Extractables Handbook: Safety Evaluation, Qualification, and Best Demonstrated Practices Applied to Inhalation Drug Products (Wiley,Hoboken, NJ, USA, 2012, pp. 185-216).
- Ball, D.J.; Beierschmitt, W.P.; Shaw, A.J. Pharmaceutical Container Closure Systems: Selection and Qualification of Materials. Ibid, pp. 217-240.
- Paskiet, D.; Jenke, D.; Ball, D.; Houston, C.; Norwood, D.L; Markovic, I. The Product Quality Research Institute (PQRI) leachables and extractables working group initiatives for parenteral and ophthalmic drug products (PODP). PDA J Pharm Sci Tech. 76(5): 430-477 (2013).
- Jenke, D. et al. Extractables characterization for five materials of construction representative of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Tech. 76(5): 448-511 (2013).
- Jenke, D.; Ruberto, M. Correlating material composition to extractables and then to leachables. In Conferences Proceedings, Extractables & Leachables USA 2013. (Smithers Information Ltd, Shropshire, UK, 2013, paper 14).
- Jenke, D. Extractable/Leachable substances from plastic materials used as pharmaceutical product containers/devices. PDA J Pharm Sci Technol. 56(6):332-371 (2002).
Dr. Dennis Jenke is a Baxter Distinguished Scientist at Baxter Healthcare Corporation. He has published extensively in the areas of analytical chemistry, environmental science and material/solution compatibility, serves as an expert reviewer for pharmaceutical and analytical journals, is a member of professional and standard-setting organizations whose charter is to establish best demonstrated practices for material/ solution compatibility and is a frequent speaker on the subject of material/solution compatibility. He authored the book “Compatibility of Pharmaceutical Solutions and Contact Materials; Safety Considerations Associated with Extractables and Leachables”. Dr. Jenke can be reached at [email protected]
Dr. Michael Ruberto is the President of Material Needs Consulting, LLC which provides consulting services to manage the development and commercialization of medical devices and packaging, emphasizing material selection, extractables and leachables, and supply chain management. He is an active member of organizations that have developed best practices for characterizing and evaluating container closure systems and packaging including the PQRI Orally Inhaled and Nasal Drug Product E&L Working Group, the PQRI Parenteral and Ophthalmic Drug Product E&L Working Group and the United States Pharmacopeia (USP) Packaging and Storage Expert Committee. Dr. Ruberto can be reached at michael.ruberto@ materialneedsconsulting.com