Diane Farrugia's research was carried out as part of the Master of Science in Industrial Pharmacy with the department of Pharmacy, Faculty of Medicine and Surgery, University of Malta, Msida. This research was carried out under a confidentiality agreement and a declaration of interest with the Malta Medicines Authority.
Introduction
The EU Good Distribution Practice (GDP) guidelines apply to manufacturers, wholesalers and other actors involved in the distribution of medicinal products. The revised GDP guidelines of the European Union were published on 8 March 2013 and became effective six months later, nineteen years after the previous publication of 1 March 1994. The revised guidelines brought about the much-needed changes in requirements to better reflect the complex distribution networks in supply chains and to be in line with Directive 2011/62/EU to prevent falsified medicinal products from entering the legal supply chain. These guidelines were later replaced by the 2013/C 343/01 guidelines of 5 November 2013 in order to correct two factual mistakes.
GDP Inspections
In the European Economic Area (EEA), wholesale dealers of medicinal products for human use need to abide by the EU legal framework in order to safeguard patient health and ensure quality, safety and efficacy of the product. Directive 2011/62/EU which amends Directive 2001/83/ EC, states that ‘manufacturers, located in the Union or in third countries, and wholesale distributors of medicinal products shall be subject to repeated inspections’1 by the competent authority of the Member State concerned. In Malta, the Inspectorate and Enforcement Directorate within the Malta Medicines Authority conduct these inspections,2 where inspection findings for GDP are in relation to: Directive 2001/83/EC3 as amended; the EU GDP guidelines 2013/C 343/01;4 Malta Medicines Act Chapter 4585 and Subsidiary Legislation 458.37.6 The aim of this research was to analyse GDP inspection reports, identify deficiencies and trend data for pharmaceutical wholesale dealers for human use in Malta and provide an insight into areas that require improvement.
Data Collection and Analysis
A retrospective study was carried out, by reviewing the last full GDP inspection report of all current pharmaceutical wholesale dealers of medicinal products for human use in Malta. Full GDP inspections consider all areas of the EU GDP guidelines and are intended to issue or renew a wholesale dealer’s licence. Number of wholesale dealers used in this study amounted to 78 (N = 78). All business models are therefore represented in this study.
Findings were assigned as Critical, Major and Other deficiencies as obtained from the inspection report in accordance to the Compilation of Community Procedures on Inspections and Exchange of Information, which is ‘a tool for facilitating co-operation between the GMP inspectorates of the Member States and a means of achieving harmonisation’7 within the European Economic Area. Findings were also categorised according to the relevant chapter and section of the revised EU GDP guidelines.
Information regarding dates of inspections from 2013 onwards was collected to identify if the last inspection was the initial, first or second inspection since the introduction of the revised EU GDP guidelines. Data analysis was then carried out, to calculate the frequency by which deficiencies in each of the sections were encountered and identify issues.
Results
Three inspections have been reported to have Critical deficiencies (N = 10). Critical deficiencies were related to Premises and Equipment (n = 6), Storage (n = 1), Documentation (n = 1), Training (n = 1), and Falsified Medicine (n = 1).
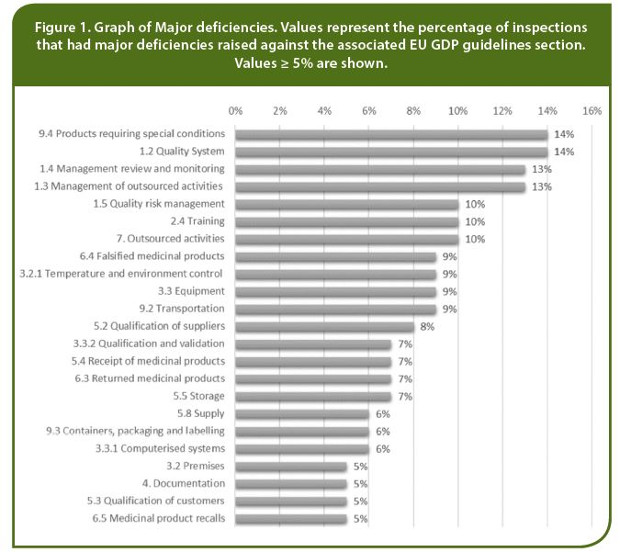
On the other hand, Major deficiencies (N =132) have been assigned to nearly all sections of the EU GDP guidelines. It was found that 44% (n = 34) of wholesale dealers were assigned one or more Major findings. These Major deficiencies were related to the EU GDP guidelines sections as presented in Figure 1. Quality Management (Chapter 1) was found to be the chapter of main concern, with a total of 23% (n = 18) of wholesale dealers having a Major deficiency in one or more areas of this chapter. For wholesalers distributing and handling cold chain medicinal products or other medicines that require special storage conditions (n = 35), five Major deficiencies were identified related to Section 9.4 regarding the transportation of products with special conditions.
All 78 wholesale dealers had Other deficiencies (N = 633). The top three Sections were wholesale dealers were assigned Other deficiencies were: Quality System (54%, n= 42), Other personnel (48%, n = 35), and Training (47%, n = 37).
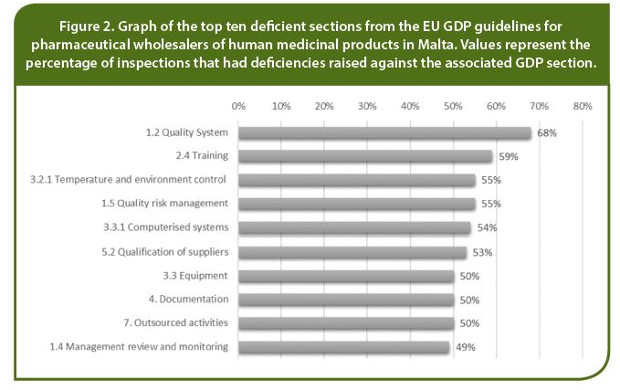
An analysis of the top ten deficient sections from the EU GDP guideline (including all Critical, Major and Other deficiencies) is presented in Figure 2, while Table 1 provides a summary of results for all inspections combined, and by inspection category: initial inspections, first inspections and second inspections following the revised EU GDP guidelines.
From studies carried out in the Netherlands on the Lessons Learned from Continued GDP Inspections in the Netherlands Based on the New EU GDP Guidelines by Bruinink and Bishara (2018)8 and United Kingdom as reported from the MHRA GDP Inspection Deficiency Data 2016 by Brown, Madigan, and Ault (2017)9 it is evident that Major deficiencies were predominantly related to the Quality Management System. Bruinink and Bishara report that 22 out of the 56 Major deficiencies (39%) were related to quality management issues while the MHRA report that the most common issues were related to the quality system with 22 % of all wholesale dealers having Major deficiencies raised against Section 1.2 Quality Systems.
Areas for Improvement
The revised EU GDP guidelines clearly specifies that ‘a responsible person should be appointed by the management, who should have clearly specified authority and responsibility for ensuring that a quality system is implemented and maintained’.4 This study shows that several areas require more attention for better compliance, especially in relation to the new requirements introduced in this revised EU GDP guidelines. Abiding by these requirements necessitate knowledge and experience which seemed to be lacking in several important areas of the EU GDP guidelines especially with respect to:
- Managing a quality system.
- Conducting risk assessments.
- Carrying out audits on service providers.
- Preparing written contracts.
- Qualification of suppliers and customers.
- Providing appropriate training to relevant personnel.
- Ensuring that all systems are in place in the event of encountering falsified medicine or in case of theft of medicinal products.
- Ensuring that temperature mapping has been properly done and reported.
- Ensuring that temperature monitoring is adequate, and equipment calibrated as required.
- Ensuring that transportation routes, vehicles and containers are appropriate and that the integrity of the medicinal products is not compromised.
- Ensuring that computerised systems are in line with the requirements of the guidelines.
- Practicing good documentation.
References
- European Commission. Directive 2011/62/EU of the European Parliament and of the Council of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products (Text with EEA relevance) [Online]. Official Journal of the European Union 2011; L 174/74-87 [cited 2018 May 15]. Available: https:// ec.europa.eu/health/sites/health/files/files/eudralex/vol- 1/dir_2011_62/dir_2011_62_en.pdf
- Malta Medicines Authority [Online]. San Ġwann: Medicines Authority; 2018 [cited 2018 August 30]. Available: http://www.medicinesauthority.gov.mt/ gooddistributionpractice#Good%20Distribution%20 Practice%20Inspections
- European Commission (EC). Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use [Online]. Brussels: EC; 2012 [cited 2018 May 5]. Available from: https://ec.europa. eu/health/sites/health/files/files/eudralex/vol-1/ dir_2001_83_consol_2012/dir_2001_83_cons_2012_ en.pdf
- European Commission. Information from European Union Institutions, Bodies, Offices and Agencies, European Commission Guidelines of 5 November 2013 on Good Distribution Practice of medicinal products for human use (Text with EEA relevance) (2013/C 343/01) [Online]. Official Journal of the European Union 2013; C343/1-14 [cited 2018 May 15]. Available: https:// ec.europa.eu/health/sites/health/files/files/eudralex/vol- 1/2013_c343_01/2013_c343_01_en.pdf
- Laws of Malta. Chapter 458 Medicines ACT [Online]. Valletta, Ministry for Justice Culture and Local Government [cited 2018 August 30]. Available: http://justiceservices.gov.mt/DownloadDocument. aspx?app=lom&itemid=8924&l=1
- Laws of Malta. Subsidiary Legislation 458.37, Wholesale Distribution and Brokering of Medicinal Products and Active Substances Regulations [Online]. Valletta, Ministry for Justice Culture and Local Government. [cited 2018 August 30]. Available: http://justiceservices.gov.mt/ DownloadDocument.aspx?app=lom&itemid=11275&l=1
- European Medicines Agency. Compilation of Community Procedures on Inspections and Exchange of Information [Online]. London: European Medicines Agency; 2018. [cited 2018 May 19]. Available: http://www.ema.europa. eu/docs/en_GB/document_library/Regulatory_and_ procedural_guideline/2009/10/WC500004706.pdf
- Bruinink R, Bishara RH. Lessons Learned from Continued GDP Inspections in the Netherlands Based on the New EU GDP Guidelines. Journal Pharmaceutical Outsourcing [Online]. 2018 March 20 [cited 2018 March 24]; 19(2). Available: https://www.pharmoutsourcing. com/Featured-Articles/348095-Lessons-Learned-from- Continued-GDP-Inspections-in-the-Netherlands-Based- on-the-New-EU-GDP-Guidelines/
- Brown P, Madigan T, Ault M. MHRA GDP Inspection Deficiency Data 2016. Medicines and Healthcare Products Regulatory Agency [Online]. 2017 November [cited 2018 March 24]. Available: https://assets. publishing.service.gov.uk/government/uploads/system/ uploads/attachment_data/file/667494/GDP_2016_ Deficiency_data.pdf