Introduction
As the world economy and financial markets contracted in 2009, so did the market for biopharmaceutical contract manufacturer services. As the result of this year-to-year decline, certain biopharmaceutical contract manufacturers (“CMOs”) merged or exited the business. While the short- term economic events caused dislocations for CMOs, the long-term prospects continue to be positive, as pharmaceutical and biotechnology companies continue to outsource more of their production and seek other additional services from CMOs.
In the latest report by HighTech Business Decisions, Biopharmaceutical Contract Manufacturing: Expanding Markets, New Capacities and Improved Performance, HighTech Business Decisions analysts interviewed 48 pharmaceutical and biotechnology manufacturing directors and executives from 29 biopharmaceutical contract manufacturers. The information for this article draws from interviews with 48 pharmaceutical and biotechnology manufacturing directors covering their observations about contracting the production of their biopharmaceuticals, current services outsourced, and new services that they expect to outsource.

Figure 1 - Trend of Outsourcing Budgets
Outsource Trends
Over the past years, the trends toward outsourcing production of biopharmaceuticals have shown increasing reliance on CMOs for the production of their biopharmaceuticals. Data compiled from HighTech Business Decisions’ industry studies indicate the long-term trend has been a steady increase in outsourcing over the past five years. This increase in outsourcing is shown as increasing share of manufacturing budgets devoted to outsourcing. Over the past 5 years, the share of manufacturing budgets spent on outsourced activities has increased from 50% to 75% (Figure 1). The benefits of outsourcing biopharmaceuticals have been well documented, which include lower production cost and lower capital expenditure requirements. All these factors lessen the overall project risk. Furthermore, the use of a CMO allows the client to take advantage of the production experience and know-how of the CMO. As a result of these advantages and others, the biotechnology and pharmaceutical companies continue to rely on CMOs for the production of their biologics. We see the trend continue to grow as more and more pharmaceutical and biotechnology companies continue to outsource their production needs.
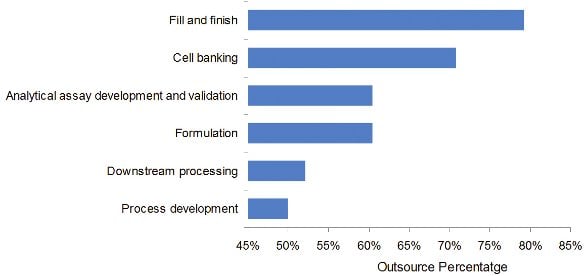
Figure 2 - Top Outsourced Services
Current/Future Need of Outsourced Services
In addition to the overall trend of outsourcing the production of biopharmaceuticals, the pharmaceutical and biotechnology companies are looking to CMOs to offer a range of other services in addition to the manufacture of their biologic API. Many of these services are closely related to the manufacturing of API, and thus are areas where CMOs have the experience and staff skills to provide these ancillary services. Furthermore, many CMOs are seeking ways to offer additional value-added services to their clients and to differentiate themselves from other suppliers. From HTBD’s study, the most widely outsourced services are a) fill-and- finish, b) cell banking, c) analytical assay development and validation, d) formulation, e) downstream processing, and f) process development. Close to 80% of the biomanufacturing directors noted that they outsource their fill-and-finish needs, while 50% of the biomanufacturing directors outsource at least some portion of process development or process improvement (Figure 2). When directors of biomanufacturing are asked about their expectation of outsourced services based on number of products to be outsourced, fill-and-finish continues to lead as the service category with the highest demand for products to be outsourced.
The biomanufacturing directors mentioned several reasons for outsourcing process development. The reason most often mentioned was the lack of expertise, or specific technology in-house, or lack of in-house capacity. On the other hand, a few of the biomanufacturing directors noted that they outsourced process development for more standard, commodity- like processes. Below are selected comments from biomanufacturing directors regarding the outsourcing of process development.
“Process development is outsourced in cases where we do not have the necessary equipment in-house or if we are lacking the skills or expertise.” Pharma/Biotechnology Company
“It depends on our internal resource requirements; it is never for different technology. We have all the techniques we need in-house. It is project prioritization, we might choose to outsource for extra capacity or hold a project back.” Pharma/Biotechnology Company
“If we think a partner has adequate capabilities, that makes a strong argument for outsourcing. We tend to outsource more in early project phases. When it gets closer to market, such as Phase III clinical trials, we tend to do it in-house and keep it under our own control.” Pharma/ Biotechnology Company
“We decide what process development projects are outsourced based on the phase and stage of the program. If we had a preclinical program that needs a lot of material, we outsource production.” Pharma/Biotechnology Company
“We did a little [outsourcing] this year, and we are looking at it again - looking forward just because of the number of projects in our pipeline. We always outsource formulation development.” Pharma/Biotechnology Company
“I think anything that is available extensively on the outside as a commodity is outsourced.” Pharma/Biotechnology Company
Important Services CMOs Can Offer
Increasingly, biomanufacturing directors seek CMOs that can offer a variety of services. In our discussions with biomanufacturing directors about CMO service, their comments are categorized either as service levels or service offerings. Service levels sought by the biomanufacturing directors include flexibility, low cost, high quality, and on-time performance. For the most part, these are service levels where the biomanufacturing directors look for continuous improvement from their CMO. For service offerings, the biomanufacturing directors look for CMOs that can offer fill-and-finish services, process development, cell line development and analytical development. Below are selected comments from biomanufacturing directors regarding service offerings by their CMO.
“The most important service a contractor can offer is the actual manufacturing of drug substance and fill-and-finish.” Pharma/ Biotechnology Company
“Because we outsource everything, that really varies. We’re not looking for someone to do an all-in-one. It’s specific to each task. Right now, it’s commitment to supply since we’re approaching commercial.” Pharma/ Biotechnology Company
“We need fill-and-finish services: syringes, vials, and lyophilization.” Pharma/Biotechnology Company
“The most important service a contractor can offer us is flexibility; they have to have the equipment we need. They need to be flexible with changes in process and timing, have strong project management, and low cost. Drug production is what we need, we crystallize the material with organics, freeze-dry it, etc.” Pharma/Biotechnology Company
"For us it would be capacity if our groups produced more than we could handle. For example, if the process development group finished more things than anticipated and we had the opportunity to press forward with manufacture, but not enough capacity, or if research provided more projects for process development than we could handle, then we might look to outsource.” Pharma/Biotechnology Company
“Cell line development is a very important service we seek and they must have access to good cell lines that will provide good yields and increase volumes as well as decrease timelines.” Pharma/Biotechnology Company
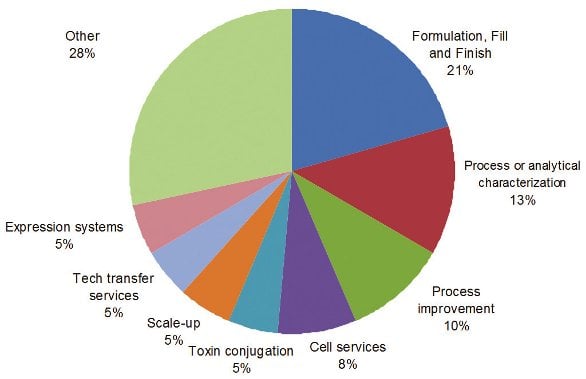
Figure 3 - New Services Sought From CMOs
New Services Sought from CMOs
In addition to the current services offered by CMOs, biomanufacturing directors are always looking for additional services from their CMOs. Product specific expertise or platforms, fill-and-finish services, process or analytical characterization, capacity, cell services and formulation are new services sought by biomanufacturing directors (Figure 3). Not surprisingly, directors from one and zero biomanufacturing site tend to be looking for greater range of new services as compared to those directors from multiple biomanufacturing sites. Below are selected comments from biomanufacturing directors.
“Fill and finish is a service that we would like from our CMO. We would prefer not to have to use a third-party.” Pharma/Biotechnology Company
“Some specialized packaging, drug delivery strategies (pre-filled syringes and other specialty packaging). There are only a few specialized companies that can manufacture and fill them. Only large companies can justify building their own fill-and-finish capabilities.” Pharma/ Biotechnology Company
“Our products are antibody drug conjugates. We are looking for a contractor that can do conjugation of potent cytotoxins to monoclonal antibodies and offers fill-and-finish services.” Pharma/Biotechnology Company
“We are looking at toxin conjugates, and some of the development may need to be done outside. That is a little different from what we normally contract for. We are also looking for analytical characterization.” Pharma/Biotechnology Company
Concluding Remarks
The overall biopharmaceutical contract manufacturing has declined from its peak in 2008 as a result of the downturn in economic activity and distressed financial markets. These short-term results are not indicative of the longer term trends. The market for outsource services will continue to expand as pharmaceutical and biotechnology companies continue to use CMOs for the production of their biologics. In addition, biomanufacturing directors are looking to their CMOs to offer other services including fill- and-finish, process development and analytical development services.
William Downey, MBA is president at HighTech Business Decisions, a consulting firm for specializing in benchmarking studies, customized market analysis and customer loyalty surveys for companies serving pharmaceutical and biotechnology markets. The company recently produced the report, Biopharmaceutical Contract Manufacturing 2009: Expanding Markets, New Capacities and Improved Performance, which is based on surveys and interviews with biomanufacturing directors at 48 pharmaceutical and biotechnology companies and 29 biopharmaceutical contract manufacturers worldwide. Mr. Downey can be reached at [email protected]

Scott M. Wheelwright, Ph.D. is president of Strategic Manufacturing Worldwide, Inc. a consultancy for biotechnology manufacturing, process development, and facilities, with particular emphasis on Asia. Dr. Wheelwright has a Ph.D. degree in chemical engineering from the University of California at Berkeley and over 20 years experience in the industry. He can be reached by email at [email protected]
This article was printed in the September/October 2010 issue of Pharmaceutical Outsourcing, Volume 11, Issue 5. Copyright rests with the publisher. For more information about Pharmaceutical Outsourcing and to read similar articles, visit www.pharmoutsourcing.com and subscribe for free.