Bioanalytical Chemistry Dept.
Overview
Technology transfer includes analytical methods, process development, and manufacturing to support biologics. The most common practice in technology transfer is analytical methods. Due to its unique complexity and variability by biological systems, cell-based potency assays are considered to be one of the most challenging parts during technology transfer. Cell-based potency assays are also considered the closest and most relevant measure of a biological mechanism of action. The current trend in the biopharmaceutical industry is to include cell-based potency assays even at earlier stages of product development. Cell-based potency assays are used for lot release and are often the early indicators of problems with product stability, impurities, and decreased potency. However, at the current moment there is no clear regulatory guidance available for cell-based potency assays.
In past years, as biopharmaceutical companies seek to increase testing capacity, free up resources for different projects, accelerate clinical timelines, and meet business continuity requirement, analytical testing performed at contract research laboratories (CRO) has increased dramatically in industry. More interest on method transfer has been accumulated. Although the most common definition for cell-based potency assays transfer is very simple: to qualify the downstream laboratory to perform the analytical procedure, the process is driven by not only business and compliance, but also technical and statistical due to the uniqueness of cell-based potency assays. Therefore, a successful transfer relies on appropriately designed processes and experiments and well-organized execution of each step. This article will discuss the challenges and key factors considered to be important for achieving successful transfer.
Effective Transfer Strategy
CRO Selection
The purpose of the transfer is to demonstrate the CRO laboratory’s proficiency in performing the particular cell-based potency assay. The first step is to select the most appropriate CRO from many candidates. The audit must be thorough and detail orientated and performed by both quality and technical subject matter expert (SME). There are many specific requirements for cell-based potency assays, such as cell culture expertise and facility, long time set up and multiple days assay, expensive instrumentation utilizing new technologies, and subtle pipetting techniques for very low serial dilutions etc. It is not easy for a CRO to build up the capability to perform cell-based potency assays proficiently. It takes time, cost and resources to gradually obtain the ability and expertise. The auditors from sponsor must fully understand the requirements for the method and then perform comprehensive review for CRO capabilities. Quality system, staff training records, SOPs, cell culture and cell bank facility, instruments, statistical software are typical contents for cell-based potency assay assessment. The sponsor should research the history and past performance of the CRO as well to ensure that the CRO meets both compliance and technical requirements.
Project Management
After CRO is selected, the project team will be composed by members from both transferring and receiving laboratories. The team should pay considerable attention to detailed role and responsibilities for each member. To identify and obtain agreement on who will execute,the outlined tasks and timelines need open and clear communication. Both transferring and receiving laboratories must be equally involved in the process. Given complexities, high variability and specialized equipment involved, it is not uncommon that assays fail during the transfer. There must be a risk evaluation and mitigation plan which should be drafted according to technical and quality assessment and CRO’s logistic support capability. The SME for trouble shooting or investigation has to be assigned at beginning. It is critical for both parties to identify any potential issues and address them appropriately up front. Setting up routine teleconferences and face-to-face meetings is recommended. Frequent exchange e-mails as a follow-up to re-define or clarify the points for clear understanding ensures the completion of outstanding items.
Documentation
Analytical method transfer can be divided as validated method transfer and non-validated method transfer which is usually in early stage of product development. Both types of methods most likely will be validated in CRO for clinical or commercial applications. So the transfer process plan has to be well documented, fully reviewed and signed by all stakeholders including quality. Furthermore, method transfer is closely related to validation, sometimes the transfer could be part of the validation. Therefore, the design for the transfer has to be articulated to downstream activities and be compliant to regulatory requirements. All protocols, SOPs, and reports should be compatible to cGXP application. All deviations, changes, non-conformance have to be recorded with justification and conclusion. Instruments and critical2011reagents have to be qualified and documented. Additionally, successful transferred method and specification for drug substance (DS) and drug product (DP) will become the final quality control procedure used for commercial production.
Detailed Experimental Design
Stages for Method Transfer
The transfer process may include training, feasibility and transfer stages. All activities have to be protocol driven with pre-determined acceptance criteria. The training can be carried out at sponsor site or trainer(s) may travel to CRO site to perform the task. After trainees understand and observe the assays demonstrated by trainers, trainees will perform training assays by themselves. The trainees could be signed off only when they are able to pass the assays consecutively. The feasibility has to be carried out at CRO. The feasibility assays will serve dual functions. One is for receiving laboratory readiness of transfer. The other is for establishment of weighting model. The transfer assays have to be performed by both sponsor and CRO analysts. Due to the inherent high variability we recommend two analysts at the transferring laboratory and two analysts at the receiving laboratory to perform cell-based potency assays transfer. The formal transfer assays will start only after the trainees feel comfortable in terms of performing the particular cell-based potency assay, as well as the weighting model is being established during feasibility stage. It is very useful to analyze historical data from the transferring laboratory and to compare to trainees results.
Sample Plan
The transferring laboratory has to develop a comprehensive sampling plan. Typical transfer should include more than one lot material for reference standard, control and test sample. The samples for the transfer experiments should be prepared in transferring laboratory and be split up two parts for two laboratories. In cell-based potency assays it is universal to apply % relative potency (RelPot) to count test sample result. The test sample could be prepared by using same concentration of reference standard, so the test sample has 100% RelPot comparative to reference standard. Since the method will be eventually validated in receiving laboratory, the additional performance characteristics such as accuracy, repeatability, linearity, stability, sensitivity etc. may not need to be evaluated in transfer. The sample plan will include the list samples such as reference standard, control, and test samples, lot number, expiration date, buffer or formulation information and storage condition etc. The certificate of analysis (COA) or batch records should be traceable. The sample shipment schedule should include the timeline, container and temperature monitoring device, documentation for chain of custody etc.
Table 1- Example for the transfer experimental design
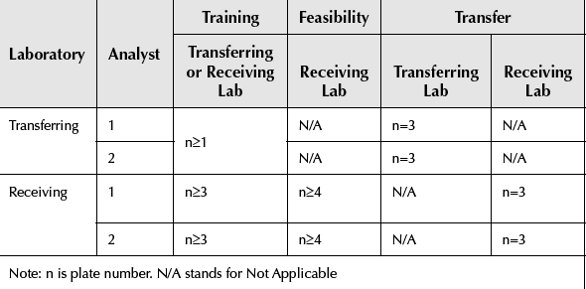
Experimental Design
The transfer experiments have to be designed as parallel as possible between transferring and receiving laboratories, so that the extra variables could be minimized. The common approach during the transfer is to perform the comparative study. A comparative study will involve measurements of test samples followed by a statisticalCROcomparison of the test results generated by transferring and receiving laboratories. The experiments for transfer should generate enough data points. Therefore, the following statistical comparison will have enough statistical power. For cell-based potency assays, the sample size for each laboratory should be ≥ 12. This means there should be at least 12 independent results. For example, if each plate could hold two test samples of sample A and sample B, in addition to reference standard and control, each sample has triplicates and each data point is the result from the average or median of the triplicates. There will be a total of 12 plates performed by four analysts. There are two analysts from transferring laboratory and two analysts from receiving laboratory. Each analyst will perform three independent plates. There are six data points for each analyst and a total of 24 data points for the transfer (Table 1). The results will be used to calculate the intermediate precision among analysts for each test sample (A and B) and means comparison by equivalence test.
One should pay extra attention to certain critical reagents for cell-based potency assays. For example, Master Cell Bank (MCB) or Working Cell Bank (WCB) has to be characterized per local appropriate regulatory requirements. The research cell bank cannot be used for transfer. Otherwise, the transferred method will not be suited for actual conditions of use.

Figure 1- Example of cell-based potency assay response full curve
System Suitability and Sample Acceptance Criteria
Most cell-based potency assays generate non-linear full curve with serial dilutions. The test sample curve will be compared to reference standard curve to pass the similarity/parallelism test first by statistical program. Then the RelPot will be calculated by measuring the horizontal difference between the sample curve and the reference standard curve at their EC50 or IC50 (Figure 1). The control curve will be compared to reference standard curve as well. The control will be used to monitor the whole assay platform/system which includes samples, reagents, plate and plate reader. The acceptance criteria for control are defined as system suitability which usually is composed of control parallelism at 95% confidence and RelPot recovery, normally 70% - 130%. It is also called assay acceptance criteria. The test sample acceptance criteria are composed of sample parallelism at 95% confidence and RelPot recovery. It is noteworthy that the definitions for system suitability and sample acceptance criteria are completely different. The assay and test sample acceptance criteria have to be determined based on accumulated historical data. Additionally, it is a continuous progress for determination of acceptance criteria. The acceptance criteria eventually will be contributed into DS and DP specifications.
Statistical Approach
Weighting Model Establishment
In cell-based potency assays the sample preparations include significant serial dilutions. The concentrations range usually from ng/mL or pg/mL to μg/mL. The concentration difference between the lowest and the highest doses can be as high as 15,000 to 20,000 fold. Therefore, it is extremely important to apply weighting model during data analysis. However, some statistical programs on the market actually do not have customized weighting functions. Therefore, it will be the sponsor’s responsibility to ensure the CRO does have the appropriate software which is not only 21 CFR part 11 compliant but also implements a customizable weighting function. During the feasibility stage, the assays should serve not only for analysts/laboratory readiness but also for estimation of weighting model. The assays for establishment of weighting model should be performed by different analysts in different days; plates should be read in different instruments using 100% RelPot reference standard as test sample. The estimated weighting model will be calculated based on dose responses (Figure 2).
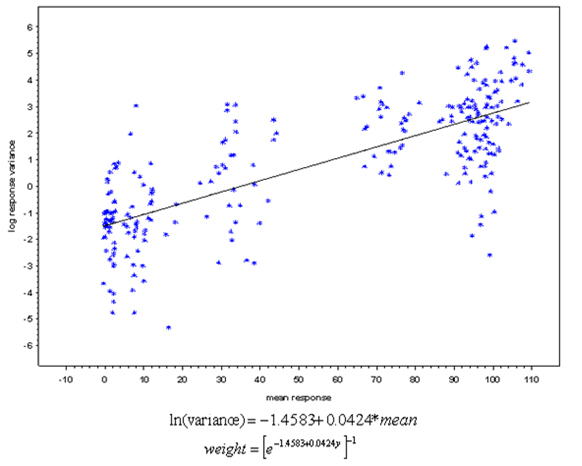
Figure 2- Example of the relationship between mean and variance
Paired Means Comparison
It is recommended to use equivalence test to compare the means generated by analysts from transferring and receiving laboratories respectively. The individual data points will be contributed into two means. The question is whether there is a significant difference between the two means from transferring and receiving laboratories respectively after we are satisfied for the variance among analysts for each test sample. In other words, if there is bias between transferring and receiving laboratories. The t value could be calculated using the following formula and then be compared to a student’s t-distribution with the degree of freedom (n1+n2-2). If there is no significant difference between the two laboratories, the calculated t value from the study will be expected less or equal to t critical value.
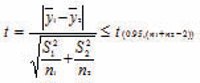
y1 bar and y2 bar are the sample means from two laboratories containing n1 and n2 data points respectively. S21 and S22 are two data groups variance from two laboratories respectively. t(0.95, (n1+n2-2)) is the 95th percent from a t-distribution with (n1+n2-2) degrees of freedom.
Troubleshooting A statistical reviewing process of assay results must be implemented. All assays performed in both transferring and receiving laboratories have to be in assay trending program (ATP). The shewhart control chart is a very valuable tool to monitor assay parameters such as RelPot, parallelism test stat values (F or Chi-square), recovery, EC50 or IC50, Max or Min, S/N etc. If any assay or sample does not pass acceptance criteria the cause must be identified. When assays consistently fail, the SME from transferring laboratory has to initiate investigation immediately and start to explore the root cause. Because of the inherent variability in cell-based potency assays, it is critical to employ statistical methods to design experiments and analyze data. The statistical analysis such as trending/regression, correlation analysis among critical parameters, and location significance test is a very power tool for data mining and troubleshooting.
Summary
Technology transfer, especially for cell-based potency assays are very complicated and challenging. The key steps for transfer of cell-based potency assays have been described above. This article also discusses the risks and pitfalls associated with transfer of cell-based potency assays. Selection of appropriate CRO, application of effective transfer strategy, design-detailed experiments with pre-determined acceptance criteria, close monitoring and timely communication, meeting of regulatory requirements and utilization of statistical tools are critical for successful transfer of cell-based potency assays.
Liming Shi (M.A., M.Sc.)is a senior scientist within Bioanalytical Chemistry Department at Amylin Pharmaceuticals Inc., in San Diego, CA. He specializes in bioanalytical methods; especially cell-based assays development, transfer, and validation. With thorough statistical expertise, intensive GMP experience and bioanalytical knowledge, he has successfully managed numerous bioanalytical projects to support studies in different phases. He has been deeply involved in PK analysis, immunogenicity assessment and CMC activities to support IND and BLA submissions.