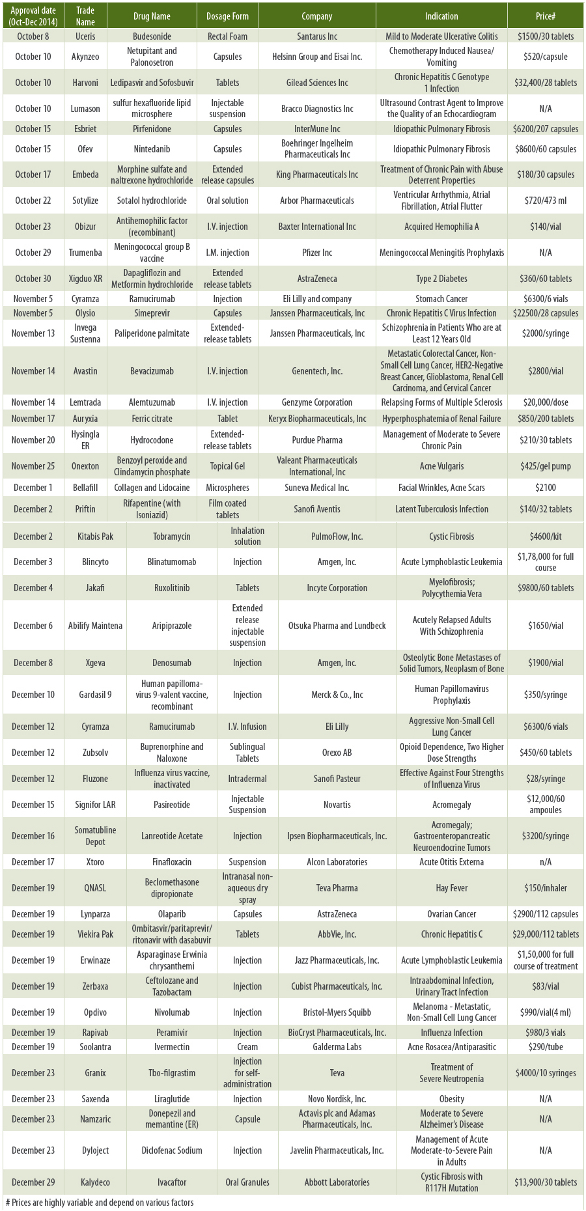
This column summarizes New Drug Applications (NDAs) for the Fourth quarter 2014 (October through December 2014). In this quarter, FDA approved 46 NDAs.
Thirty (65.2%) NDAs were for small molecules while the remaining 16 molecules (34.8%) were large molecules. Similar to the previous quarter, companies are getting interested in working on the large molecules. In this quarter, 22 companies (47.8%) receiving NDA approvals were large companies and the remaining 24 companies (52.2%) were midsized and small companies. Tablets (15.2%) and Injections (39.1%) were the preferred dosage forms. Eleven out of 46 (23.9%) of NDAs were combination dosage forms.
Some drugs have unique properties. Embeda has properties that are expected to reduce, but not totally prevent, abuse of the drug when crushed and taken orally or snorted. Embeda works by releasing only the morphine in the capsule when taken properly. When crushed, the naltrexone in Embeda blocks some of the euphoric effects of the morphine and can precipitate withdrawal in persons dependent on opioids. Amgen received FDA’s Breakthrough Therapy Designation for Investigational BiTE antibody Blinatumomab for acute lymphoblastic leukemia on July 1, 2014. It received a priority review designation on October 9, 2014. Kaydeco received the priority review designation on December 15, 2011. It received an approval on January 31, 2012 to treat a rare form of cystic fibrosis. Lynparza (olaparib) is a first-in-class oral poly ADP ribose polymerase (PARP) inhibitor for the treatment of advanced ovarian cancer. It received an accelerated approval in June 25, 2014. FDA approved Trumenba (meningococcal group B vaccine), the first vaccine licensed in the United States to prevent invasive meningococcal disease caused by Neisseria meningitides serogroup B in individuals 10 through 25 years of age.
Many formulations that were previously approved by the FDA have received approvals for new indications in this quarter. FDA had approved Jakafi to treat myelofibrosis on November 16, 2011. FDA approved Xgeva to treat Giant Cell Tumor of the bone on Jun 13, 2013.
FDA approved Xgeva for the prevention of skeletal-related events in patients with bone metastases from solid tumors on November 19, 2010. Cyramza was first approved on April 21, 2014 for stomach cancer. It was approved in combination with paclitaxel for advanced gastric cancer after chemotherapy. Somatuline Depot received marketing approval for the treatment of acromegaly on August 31, 2007. Santarus received FDA approval of Uceris (budesonide) for the induction of remission in patients with active, mild to moderate ulcerative colitis on January 15, 2013. FDA approved Embeda for the management of moderate to severe chronic pain on August 13, 2009. FDA approved Olysio (simeprevir) for Hepatitis C virus on November 24, 2013. FDA Approved Olysio (simeprevir) in combination with Sofosbuvir for Genotype 1 Chronic Hepatitis C Infection on November 5, 2014. FDA approved Invega Sustenna for the acute and maintenance treatment of schizophrenia on August 3, 2009. Invega was approved as the first and only treatment for schizoaffective disorder on August 6, 2009. Invega was approved as the treatment for schizophrenia in adolescents on April 11, 2011. FDA approved Avastin in combination with chemotherapy for first-line treatment of most common type of lung cancer on October 11, 2006. FDA granted accelerated approval of Avastin in combination with paclitaxel chemotherapy for first-line treatment of advanced HER2-Negative breast cancer on February 25, 2008. FDA granted accelerated approval of Avastin for Brain Cancer (Glioblastoma) that has progressed following prior therapy on May 6, 2006. FDA approved Avastin for the most common type of kidney cancer on August 3, 2009. FDA Approved Avastin for metastatic cervical cancer on August 14, 2014. Keryx Biopharmaceuticals received FDA approval of Ferric Citrate for dialysis patients with Hyperphosphatemia on September 2104. Keryx Biopharmaceuticals announced trade name Auryxia for Ferric Citrate on November 17, 2014. FDA Approved ArteFill as the first non-resorbable injectable wrinkle filler to correct smile lines on October 30, 2006. They changed the name of Bellafill on December 1, 2014. This dermal filler is comprised of 80 percent purified bovine collagen with 20 percent polymethyl-methacrylate (PMMA) microspheres, and lidocaine.