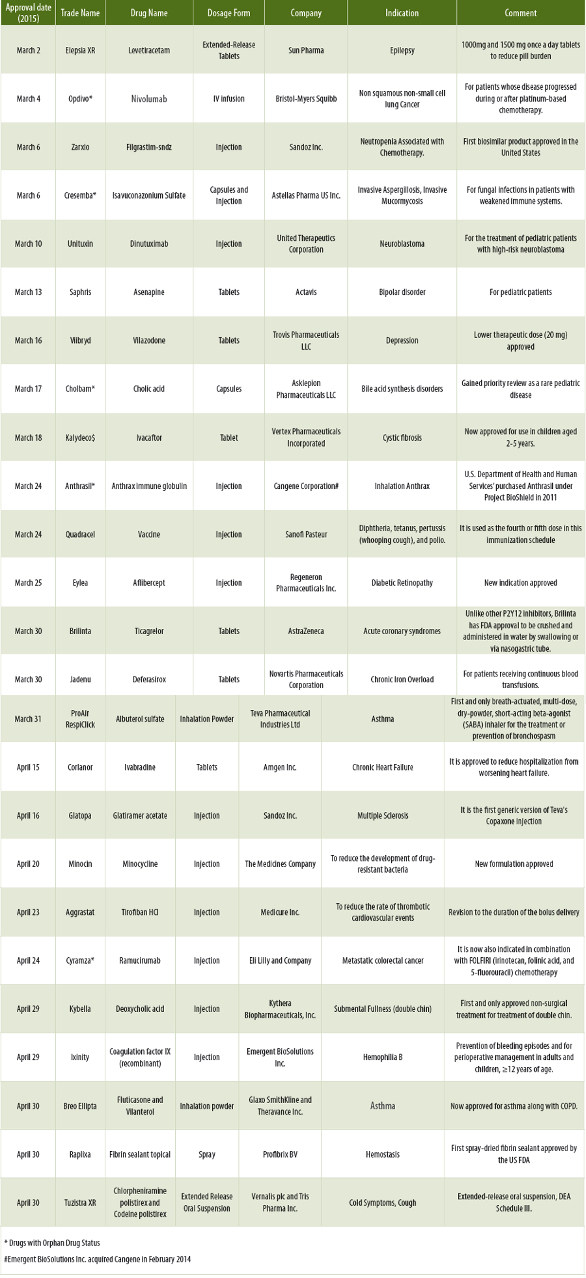
This column summarizes New Drug Applications (NDAs) for March and April 2015. In these two months, FDA approved 25 NDAs.
Sixteen (64.0%) NDAs were for small molecules while the remaining 9 molecules (36.0%) were for large molecules. Most of the products containing large molecules involved antibodies and related molecules for the treatment of different cancers. A total of 12 companies (48.0%) receiving NDA approvals were large companies and the remain-ing 13 companies (52.0%) were mid-sized and small companies. Six drug molecules were granted the Orphan Drug status (24.0%). Tablets and Injections were the preferred dosage forms. A spray (Raplixa) was developed by ProFibrix BV consisting of fibrin sealant. Raplixa contains fibrinogen and thrombin. When applied to a bleeding site, Raplixa is dissolved in the blood and a reaction starts between the fibrinogen and thrombin proteins. This results in the formation of blood clots to help stop the bleeding. Two out of 25 (8.0%) of NDAs were combination dosage forms.
The FDA granted Qualified Infectious Disease Product (QIDP) designation for the new formulation of Minocin for Injection under the Generating Antibiotic Incentives Now Act (GAIN Act). The designation, the third granted to a product in the company’s infectious disease portfolio, qualified Minocin for Injection for priority review and five years of marketing exclusivity upon an approval of the additional potential indications.
Zarxio by Sandoz Inc. is a biosimilar product to Amgen’s Neupogen (filgrastim), which was originally licensed in 1991. A biosimilar product is a biological product that is approved based on a proof showing that it is highly similar to an already-approved biological product, known as a reference product. The biosimilar also must show that it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable in biosimilar products. Zarxio is approved for the same indications as Neupogen.
Jadenu developed by Novartis consists of Deferasirox, which represents a new class of tridentate iron chelators.
The new approved labeling of Aggrastat allows the delivery duration of high-dose bolus (25 mcg/kg) to occur anytime within 5 minutes, instead of the previously specified duration of 3 minutes.
FDA has withdrawn its approval of the NDA for Elepsia XR due to regulatory issues at the manufacturing plant of Sun Pharma. The company is working with the FDA to resolve the manufacturing issues. Cresemba is used to treat adults with invasive aspergillosis and invasive mucormycosis. These infections occur most often in people with weakened immune systems. This azole antifungal agent targets the cell membrane of fungus. Unituxin (dinutuximab) is part of first-line therapy for pediatric patients with high-risk neuroblastoma, a type of cancer that most often occurs in young children. Unituxin is an antibody that binds to the surface of neuroblastoma cells. Healthcare professionals should be aware of the risk of Type I hypersensitivity reactions with Saphris, the drug for bipolar disorder. Anthrasil is an integral part of the U.S. government's strategic national stockpile and has received Orphan Drug designation. As a result of this approval, the product qualifies for seven years of market exclusivity. Anthrasil is manufactured from the plasma of individuals vaccinated against anthrax. Eylea (aflibercept) is made from a human antibody fragment. It works by keeping new blood vessels from forming under the retina. Tirofiban (trade name Aggrastat) is an antiplatelet drug. It belongs to a class of antiplatelet named glycoprotein IIb/IIIa inhibitors. Tirofiban is the first drug candidate whose origins can be traced to a pharmacophore-based virtual screening lead.